Chart of the Week: How Data Exclusivity Laws Impact Drug Prices
By Rachel Thrasher
As a recent working paper by Michael Palmedo shows, countries that have enacted data exclusivity into their intellectual property laws have faced increased pharmaceutical import prices over the past 20 years: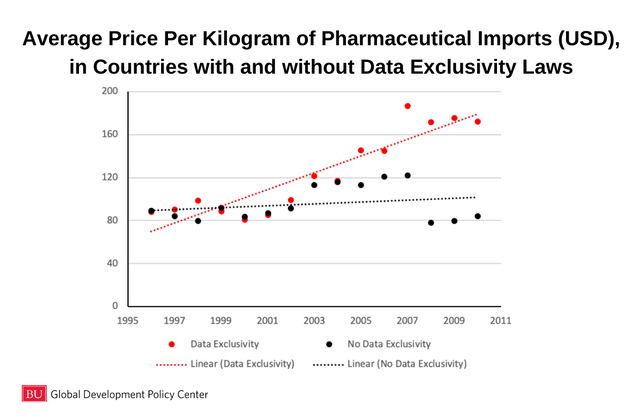
Data exclusivity is a form of intellectual property protection that applies specifically to data from pharmaceutical clinical trials. While innovator firms run their own clinical trials to gain marketing approval, generic manufacturers typically rely on the innovator’s clinical trials for the same approval. Data exclusivity rules keep generic firms from relying on that data for 5 to 12 years, depending on the specific law. Data exclusivity operates independently of patent protection and can block generic manufacturers from gaining marketing approval even if the patent has expired or the original pharmaceutical product does not qualify for patent protection.
Although data exclusivity laws are matters of domestic legislation, the United States, the EU and others increasingly demand in their free trade agreement (FTA) negotiations that their trading partners protect clinical trial data in this way. Data exclusivity is just one of a host of “TRIPS-plus” treaty provisions designed to raise the overall level of intellectual property protection for innovator firms. Although the WTO’s Agreement on Trade-Related Intellectual Property Rights (TRIPS) does require Member states to protect clinical trial and other data from “unfair commercial use,” it does not require exclusivity rules that block the registration of generic products.
“T
Palmedo’s research conclusively shows that data exclusivity, a measure required in all FTAs with the United States, is associated with increased prices across pharmaceutical imports for 41 countries. Countries on the brink of initiating negotiations with the United States should be on notice that the TRIPS-plus rules preferred by the United States negotiators may have real impacts on the affordability and accessibility of medicines for their constituents.
Read the Working Paper