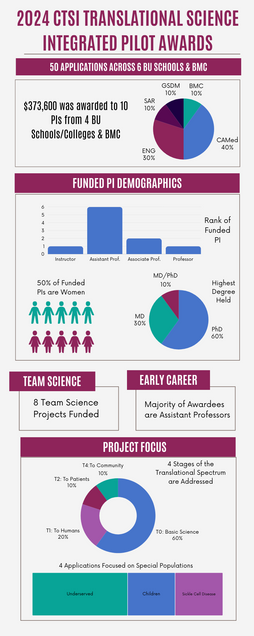
The CTSI provides pilot funding for innovative translational research and the development of research methods at BU. That encompasses the continuum from the development of new therapies and diagnostic tests, to studies of the population health impact of health interventions.
The overarching goal of the BU CTSI Pilot Grant Program is to help investigators explore and solve major challenges in translational science, especially those that address the special health problems of our urban communities, by developing and deploying new tools, methods, and processes to expedite clinical and translational research and discovery.
We seek to stimulate individual and team science in all areas of translational research related to the prevention, diagnosis, and management of the human disease. Researchers engaged in translational basic/bench, clinical, biomedical, patient-oriented, implementation, and population health science research are encouraged to apply. The CTSI welcomes applications that are at all levels of the translational continuum including:
T0 research that generates foundational knowledge and insights by bridging the gap between exploratory studies and basic scientific discoveries, leading to the development of new hypotheses and scientific investigation.
T1 research that develops novel treatments and interventions by expediting the movement between basic research and patient-oriented research leading to new or improved scientific understanding or standards of care.
T2 research that tests the efficacy and effectiveness of interventions through patient-oriented research and population-based research leading to better patient outcomes, the implementation of best practices, and improved health status in communities.
T3 research that promotes dissemination and implementation of research for system-wide change through the movement of evidence based-guidelines into clinical practice.
T4 research that promotes discoveries in population science.
Pilot Information Session
Priority Areas of this RFA
This RFA is open to ALL members of the CRC or BMC/BU community and represents a collaborative effort between the CTSI and partner organizations to fund meritorious research applicable to at least one (or more) of the components noted below:
1. CTSI General Funds
Eligible faculty may apply to this component. While all areas of investigation are eligible, the CTSI is particularly interested in supporting research that involves special populations served by BMC/BUMC including (but not limited to) children and adolescents; the elderly; underserved and low socioeconomic status (SES) patients; as well as and diseases that affect patients across their lifespan.
Priority will be given to applications that focus on Translational Science (https://ncats.nih.gov/training-education/translational-science-principles), such as projects that address challenges and roadblocks to advancing translational progress, innovative to accelerate the pace of translational research, and projects that are applicable to multiple diseases/conditions.
2. Community-Engaged Research
Eligible faculty may apply to this mechanism. These projects must be designed to stimulate community-academic partnerships with the goal of catalyzing innovative translational research that is responsive to community health needs. We define community as the diverse, under-resourced populations served by Boston Medical Center and its affiliated community health centers.
3. Bioinformatics/Mobile Health
Eligible faculty may apply to this component. These projects must leverage existing bioinformatics resources or mobile health applications to facilitate improved health outcomes, facilitate access to care, or address health disparities among vulnerable populations.
4. BU Chobanian & Avedisian School of Medicine (CAMed)
Faculty whose grants are submitted through CAMed (BU Chobanian & Avedisian School of Medicine) are eligible to apply for this funding mechanism. Priorities will be for multi-investigator d collaborative applications.
5. Henry M. Goldman School of Dental Medicine (GSDM)
This funding element specifically funds meritorious applications from GSDM faculty.
6. Evans Center for Biomedical Research
The CTSI will support new research programs that align with existing Affinity Research Collaboratives (ARCs) priorities supported through the Evans Center for Interdisciplinary Biomedical Research (http://www.bumc.bu.edu/evanscenteribr) including non-ARC affiliated investigators with research aligned with an existing ARC, innovative research from ARC investigators and proposals that support the development of new ARCs.
This element is intended to:
• Fund projects from non-ARC affiliated investigators that facilitate entry into an established Evans Center ARC
• Fund projects from current ARC investigators that expand a current ARC with an innovative direction
• Fund projects from any investigator that contributes to the development of a new ARC in the following year
7. Department of Medicine (DOM)
Faculty members with primary appointments in the Department of Medicine are eligible to apply for this mechanism. All meritorious T1-T4 applications will be considered.
8. Addiction Science Research
Eligible faculty focused on addiction sciences may apply to this mechanism. Addiction science is a priority of the CTSI and is in part supported by partners including the BMC Grayken Center and SPH Alcohol Addiction Center.
9. New Product/Device Development
CTSI encourages the development and evaluation of new products, devices, or drug targets that have commercial potential. While CTSI will support applicants at any stage of the development timeline, this mechanism is best suited to early feasibility studies and/or proof of concept. Applicants will be connected to institutional resources to support this process.
Eligibility
Applicants must have a primary faculty appointment at Boston University, and the research to be conducted must be based at Boston University, Boston Medical Center or any of their affiliated hospitals and health centers. Faculty with co-appointments at BU and BU/BMC affiliated institutions are also encouraged to apply. Please see above for additional eligibility requirements based on the component to which the applicant is applying. In particular we encourage early investigators to apply, including those with institutional or individual mentored research awards from NIH or Foundations.
Recipients of previous CTSI awards are eligible to apply for awards to support new research projects, providing awards are at least two years apart with evidence of successful completion and productivity with the prior award.
Individuals from under-represented groups are highly encouraged to apply.
Applications from interdisciplinary teams of investigators are highly encouraged (see below for definition). Collaborations with investigators outside of the BU/BMC and affiliates are allowed, though justification should be provided. To search for BU collaborators, refer to BU Profiles or send a request to the BU CTSI Navigator team at ctsisvcs@bu.edu.
Funding Levels
Two award levels are offered:
- 1. Direct costs up to $20,000 may be requested for applications with a single PI
2. Direct costs up to $50,000 may be requested for applications with 2 or more investigators comprising a credible research team (funding at this level will be subject to resource availability). Interdisciplinary teams comprised of investigators from different Sections, Departments, Schools, Institutions, or campuses will be prioritized.
The level of funding awarded to successful applicants will be determined after a review of the budget request and budget justification. Funds may be used for any purpose to support the proposed research. Typical expenses include:
- Laboratory supplies
- Animal costs
- Small equipment
- Patient recruitment costs
- Consultants
- Travel (must be specifically related to the pilot)
- Support for pre/postdoctoral students, technicians, or research assistants.
Funds may not be allocated to PI or Co-I salary. Awards are not transferable to any other institution (sub-awards are not allowed). Pilot grants are not intended to supplement existing funded awards. Significant overlap in Pilot grant Aims with Aims from a funded grant must be disclosed and justified at the time of submission.
Research Proposed in response to this RFA must be accomplished within the specified award period.
Requirements for Regulatory Approval
IRB and IACUC Approvals: All IRB and IACUC protocols must be approved prior to the expenditure of any funds.
Delayed Onset Human Subjects Research: The NIH requires that the CTSI obtain explicit approval from the NIH for any CTSI-funded pilot with research involving human subjects. Accordingly, the IRB-approved protocol and other materials must be submitted to the NIH at least 45 days prior to the project start date. CTSI personnel will work with awardees to meet these requirements for those pilots that are funded with CTSI grant funds.
Prior Approval of Vertebrate Animals Research:
The NIH requires that the CTSI obtain explicit approval from the NIH for any CTSI-funded pilot with research involving vertebrate animals. IACUC approval documentation and other materials must be submitted to the NIH at least 45 days prior to the project start date. CTSI personnel will work with awardees to meet these requirements for those pilots that are funded with CTSI grant funds.
We strongly advise all Pilot grantees to begin the IRB/IACUC approval process at the time of final grant submission. If you are selected for funding, you will be required to provide your IRB/IACUC approval before funds are released to you.
If, at the time of notice of award, you have not submitted your IRB/IACUC application, you will be required to meet with a CTSI IRB/IACUC consultant within 2 weeks and expected to follow a strict timeline for regulatory submission. An award may be rescinded for failure to secure IRB/IACUC approval 3 months after notice of award.
Due to regulatory restrictions around awards that involve Human Subjects Research, please ensure that the title of your pilot application matches your IRB application. The title of the application must match the title on the IRB outcome letter.
Application Submittal Process
All applications can be submitted here and must include:
- A maximum of three pages describing the background, specific aims, preliminary studies (if applicable), research methods (including timeline), commercial potential, and future grant submissions or commercial development. The application must state how all requirements for receipt of the award have been addressed.
- Current NIH formatted biosketch (s)
- A budget & budget justification
- Other relevant supporting documentation (optional) may also be included if it’s relevant to the application
Applications will be reviewed by the BU CTSI Scientific Review Committees comprised of faculty with relevant expertise analogous to the NIH review process (with a focus on Significance, Innovation, Approach, and Investigators). Specific review criteria include, but are not limited to:
- Significance of the work in terms of potential health impact
- Scientific rigor and novelty of the proposed approach
- Likelihood that the project will lead to subsequent external funding and/or commercial development
- Multidisciplinary collaboration
- High potential for impact in the prevention, diagnosis, or treatment of human health conditions in broad terms. Projects closer to translation will be prioritized over more preliminary projects that are further from translation.
- Qualification of the research team
- Need for the funding
- Likelihood that the project can be completed within the awarded project period
- Although not required that applicants be early-stage investigators, the review process will consider career development.
- Although not required, other features that may increase a project’s priority include:
• A clear translational focus, including a collaboration with a patient-oriented science research team
• Focus on diseases that disproportionately affect the BU/BMC patient population or ages at the extremes of the lifespan (children and the elderly)
• Approved IRB or IACUC protocols that would permit initiation of research activities as soon as possible
Available Resources
Available Resources for the Pilot Grant Application
The CTSI offers an array of research resources in support of research at BU and we strongly encourage CTSI Pilot Grant applications to use these valuable resources. In many cases, leverage of these resources increases the competitiveness of an application. Some examples include the following:
- Regulatory Support
- Grant Writing & Editing, Formatting and Editing Services
- Biostatistics, Data Management & Analysis
- Research Tools
- Study Implementation
- Research Networking
- Consultations – CTSI staff will offer feedback on the feasibility, impact, and design of the proposed scientific investigations
To request a CTSI free service please visit the CTSI Research Navigator Team page here.
Post Award Requirements
If funded, the awardee agrees to submit an online report after the end of the award term indicating key results and any publications, grant applications, funded awards that resulted from the project, new collaborations, and other outcomes. This report must be completed on time if a 2nd stage renewal application is being pursued. Additional abbreviated reports will be requested on an annual basis for 2 years following completion of funding. Any follow on funding depends on prompt and accurate progress reports. Awardees will be contacted regarding the report, once needed.
Awardees must acknowledge the CTSI grant in any publication or presentation that arises from data collected through this CTSI-funded award, the language provided below is recommended:
“This publication [or project] was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Boston University Clinical & 转化科学澳门威尼斯人注册网站研究所 Grant Number 1UL1TR001430. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.”
Awardees are expected to serve on future CTSI Pilot Grant Application Review panels and to provide feedback on the CTSI pilot program. They may be asked to participate in CTSI-related symposia or other functions. Awardees may be matched with relevant CTSI functions to support regulatory requirements or completion of proposed Aims and are expected to follow through on suggested timeliness and meetings with CTSI staff.
Failure to comply with all post-award requirements jeopardizes eligibility for future Pilot award funding.
Where to Direct Inquiries
We encourage inquiries concerning this RFA and welcome the opportunity to answer questions from potential applicants. Direct your questions to:
| Elisha Wachman, MD Department of Pediatrics Co-Director, Pilot Grant Program, BU CTSI E-mail: Elisha.Wachman@bmc.orgAndrew Henderson, PhD Department of Medicine, Infectious Diseases Co-Director, Pilot Grant Program BU CTSI E-mail: hender@bu.edu |
Administrative Contact: Hubert Wong Director of Finance & Operations, BU CTSI 617.358.7553 (ph) ctsipilots@bu.edu |
2024 Integrated Pilot Grant Awardees
Funded by BMC |
Placental Repository to Improve Infant Lung Outcomes in Preeclampsia
The BMC PRIME study is a longitudinal prospective study evaluating placental secreted proteins from preeclamptic pregnancies. Preeclampsia is a hypertensive disorder of pregnancy that is strongly associated with neonatal lung and gut disorders for premature infants. Through the PRIME study, Dr. Taglauer will seek to identify unique signaling proteins from preeclamptic placentas that are connected with pathways of lung and gut developmental injury. The PRIME study will also be creating a highly valuable biorepository of preeclamptic placental samples that will be available for collaborative studies.
 Dr. Elizabeth Taglauer is a neonatologist and placental biologist who has developed a unique niche as a physician-scientist to explore how the intrauterine environment can be optimized to improve neonatal outcomes. In her faculty research at Boston Medical Center, Dr. Taglauer is applying her placental expertise to examine how perinatal exposures impact neonatal outcomes. The main focus of her work is on the antenatal determinants of neonatal lung and gut disease, seeking to understand how human placental dysregulation impacts fetal lung and gut development. Dr. Taglauer’s research incorporates human placental components with translational human developmental model systems with a long-term goal of optimizing infant development and health outcomes.
Dr. Elizabeth Taglauer is a neonatologist and placental biologist who has developed a unique niche as a physician-scientist to explore how the intrauterine environment can be optimized to improve neonatal outcomes. In her faculty research at Boston Medical Center, Dr. Taglauer is applying her placental expertise to examine how perinatal exposures impact neonatal outcomes. The main focus of her work is on the antenatal determinants of neonatal lung and gut disease, seeking to understand how human placental dysregulation impacts fetal lung and gut development. Dr. Taglauer’s research incorporates human placental components with translational human developmental model systems with a long-term goal of optimizing infant development and health outcomes.
Funded by BU CTSI |
Validity, Acceptability, and Utility of Electronic Health Record Household Linking
艾尔
Meet the Team
Dr. Jeffrey Campbell is an Assistant Professor of Pediatrics in the Department of Pediatrics/Section of Pediatric Infectious Diseases at the Boston University Chobanian and Avedisian School of Medicine.
Dr. Karen Jacobson is an Associate Professor of Medicine in the Section of Infectious Diseases at the Boston University Chobanian and Avedisian School of Medicine and Boston Medical Center.
 Dr. William G. Adams is an epidemiologist, medical informatician, and practicing pediatrician at Boston Medical Center (BMC). He is also Professor of Pediatrics and serves as Director of BU-CTSI Biomedical Informatics for Boston University and Director of Community Health Informatics for the Boston HealthNet, an urban integrated health delivery system. His primary research is focused on developing and evaluating information technology (IT)-based solutions for improving the quality of health and healthcare for urban populations. His foci include electronic health records (EHR) for research, state-wide registries, decision support, patient-centered health IT and clinical data warehousing for quality improvement and comparative effectiveness research.
Dr. William G. Adams is an epidemiologist, medical informatician, and practicing pediatrician at Boston Medical Center (BMC). He is also Professor of Pediatrics and serves as Director of BU-CTSI Biomedical Informatics for Boston University and Director of Community Health Informatics for the Boston HealthNet, an urban integrated health delivery system. His primary research is focused on developing and evaluating information technology (IT)-based solutions for improving the quality of health and healthcare for urban populations. His foci include electronic health records (EHR) for research, state-wide registries, decision support, patient-centered health IT and clinical data warehousing for quality improvement and comparative effectiveness research.
 Dr. Jessica Haberer received her medical degree from Yale University and a master’s degree in Health Services Research from Stanford University. She trained in Internal Medicine at the University of California San Francisco and, early in her career, worked for the William J. Clinton Foundation HIV/AIDS Initiative. Since 2008, she has worked at the Massachusetts General Hospital and Harvard Medical School as a physician-researcher. Her current work focuses on HIV prevention, implementation science, and data science with collaborations primarily in East and South Africa. Dr. Haberer also serves as the Director of Research at the MGH Center for Global Health and is committed to active mentorship, promotion of diversity in the research workforce, and equity in global health research partnerships.
Dr. Jessica Haberer received her medical degree from Yale University and a master’s degree in Health Services Research from Stanford University. She trained in Internal Medicine at the University of California San Francisco and, early in her career, worked for the William J. Clinton Foundation HIV/AIDS Initiative. Since 2008, she has worked at the Massachusetts General Hospital and Harvard Medical School as a physician-researcher. Her current work focuses on HIV prevention, implementation science, and data science with collaborations primarily in East and South Africa. Dr. Haberer also serves as the Director of Research at the MGH Center for Global Health and is committed to active mentorship, promotion of diversity in the research workforce, and equity in global health research partnerships.
 Dr. Robert Horsburgh, Jr., is Professor of Epidemiology, Biostatistics, Global Health and Medicine at Boston University. He is an experienced tuberculosis (TB) clinician whose research has focused on TB epidemiology and clinical trials. He has served as Co-Chairman of the U.S. TB Trials Consortium and the U.S. TB Epidemiologic Studies Consortium. He is currently a member of the Board of Directors of the International Union Against Tuberculosis and Lung Disease and Chairman of the Steering Committee of RESIST-TB, an international organization that advocates for global expansion of treatment for Drug-resistant TB (http://www.resisttb.org/).
Dr. Robert Horsburgh, Jr., is Professor of Epidemiology, Biostatistics, Global Health and Medicine at Boston University. He is an experienced tuberculosis (TB) clinician whose research has focused on TB epidemiology and clinical trials. He has served as Co-Chairman of the U.S. TB Trials Consortium and the U.S. TB Epidemiologic Studies Consortium. He is currently a member of the Board of Directors of the International Union Against Tuberculosis and Lung Disease and Chairman of the Steering Committee of RESIST-TB, an international organization that advocates for global expansion of treatment for Drug-resistant TB (http://www.resisttb.org/).
Dr. Heather Hsu is an Assistant Professor of Pediatrics and Scientific Director of the Boston Medical Center Clinical Data Warehouse for Research.
Dr. Helen E. Jenkins‘ research focuses on the epidemiology of tuberculosis. She is interested in what we can learn about TB epidemiology using routinely-collected data sources and has collaborations in South Africa, Ukraine, and Peru. She is also interested in pediatric TB and the spatial epidemiology of TB.
Dr. Vishakha Sabharwal is a Pediatric Infectious Diseases provider at Boston Medical Center who works in the BMC TB clinic and manages and treats children with TB disease and infection. She has collaborated closely with Dr. Campbell over the past few years and most recently in the TB household linkages to study household clustering of TB infection.
A soft-foldable Robotic Eetractor with Integrated Pressure Sensing to Reduce Tissue Trauma in Neurosurgery and Skull Base Surgery
Brain retraction systems are frequently required to achieve surgical exposure of deep-seated brain lesions. Retraction, however, can cause localized stress areas and is associated with complications that include brain edema, vascular compromise causing ischemia, and direct damage to the surrounding cortex.
This pilot project will focus on the design, development, and evaluation of a novel soft robotic neurosurgical retractor to tackle current clinical barriers in neurological surgery and skull base surgery. The proposed system is designed to facilitate neurosurgery by creating a minimally invasive surgical workspace in the brain (through expansion and unfolding of pneumatically driven origami-inspired soft robotic actuators) and monitor robot/tissue interaction (via soft capacitive pressure sensors).
This soft robot provides the capability to tune the amount of tissue retraction via actively expanding and contracting its structure by controlling its internal actuation state, and continuously monitor its interaction with the surrounding environment (i.e., brain tissue) while informing the surgeon. The purpose of this device is to address critical unmet needs within the world of brain tumor surgery, such as increasing safety and the depth of access in deep-seated tumor brain locations, improving navigability within the brain, and enabling surgeon awareness during the procedure.
Meet the Team
 Dr. Sheila Russo is an Assistant Professor in the Department of Mechanical Engineering and the Division of Materials Science and Engineering at Boston University (BU). She received her Ph.D. degree at the BioRobotics Institute, Sant’Anna School of Advanced Studies, Italy. She completed her postdoctoral training at the Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. She is the founder and director of the Material Robotics Laboratory at BU. Her research interests include medical and surgical robotics, soft robotics, origami-inspired mechanisms, sensing and actuation, and meso- and micro-scale manufacturing techniques. In 2020 she received the NIH Trailblazer Award for New and Early Stage Investigators.
Dr. Sheila Russo is an Assistant Professor in the Department of Mechanical Engineering and the Division of Materials Science and Engineering at Boston University (BU). She received her Ph.D. degree at the BioRobotics Institute, Sant’Anna School of Advanced Studies, Italy. She completed her postdoctoral training at the Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. She is the founder and director of the Material Robotics Laboratory at BU. Her research interests include medical and surgical robotics, soft robotics, origami-inspired mechanisms, sensing and actuation, and meso- and micro-scale manufacturing techniques. In 2020 she received the NIH Trailblazer Award for New and Early Stage Investigators.
博士
 Dr. Urvashi Upadhyay is a board-certified neurosurgeon and director of the Brain Tumor and Skull Base Surgery Program at Boston Medical Center (BMC). Dr. Upadhyay is also a clinical associate professor of Neurosurgery at the Boston University Chobanian & Avedisian School of Medicine and the Associate Program Director for the combined BMC/Beth Israel Deaconess Medical Center (BIDMC) neurosurgery residency training program. She specializes in treating complex brain tumors, meningioma, pituitary tumors, skull base tumors, and trigeminal neuralgia. Dr. Upadhyay’s research interests include intracranial drug delivery, clinical trials in brain tumors, and device development. She collaborates with colleagues from the Massachusetts Institute of Technology (MIT) in the area of drug delivery for brain tumors and at the Material Robotics Lab here at Boston University.
Dr. Urvashi Upadhyay is a board-certified neurosurgeon and director of the Brain Tumor and Skull Base Surgery Program at Boston Medical Center (BMC). Dr. Upadhyay is also a clinical associate professor of Neurosurgery at the Boston University Chobanian & Avedisian School of Medicine and the Associate Program Director for the combined BMC/Beth Israel Deaconess Medical Center (BIDMC) neurosurgery residency training program. She specializes in treating complex brain tumors, meningioma, pituitary tumors, skull base tumors, and trigeminal neuralgia. Dr. Upadhyay’s research interests include intracranial drug delivery, clinical trials in brain tumors, and device development. She collaborates with colleagues from the Massachusetts Institute of Technology (MIT) in the area of drug delivery for brain tumors and at the Material Robotics Lab here at Boston University.
Self-Amplifying RNA Mediated Immunoglobulin Delivery for the Treatment of Breast Cancer
The goal of this project is to leverage a novel self-amplifying RNA (saRNA) technology developed by the Grinstaff and Wong lab to deliver an antibody for the treatment of breast cancer. Success from our work could provide better control of HER2-positive breast cancer progression by reducing tumor volume and extending survival compared to direct intravenous injection of chemotherapy.
Meet the Team
Dr. Wilson Wong is an Associate Professor of Biomedical Engineering at Boston University. His lab has extensive expertise in mammalian synthetic biology, RNA engineering, and immunotherapy.
Dr. Mark Grinstaff is the William Fairfield Warren Distinguished Professor, and a Professor of Biomedical Engineering, Chemistry, Materials Science and Engineering, and Medicine at Boston University. His lab is a leading pioneer in drug delivery, saRNA, nucleic acid chemistry, and biomaterials with successful translation of clinically used products (Abraxane®, OcuSeal®, and Adherus Surgical Sealants®).
A soft Robotic Catheter for Percutaneous Management of Non-compressible Torso Hemorrhage
In the US, injury accounts for over 150,000 deaths and over 3 million injuries per year. Hemorrhage, secondary to traumatic injury, is the leading cause of death of Americans from one to 46 years of age. Noncompressible torso hemorrhage is a leading cause of mortality in patients with trauma.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a minimally invasive intervention for temporary control of non-compressible torso hemorrhage originating below the diaphragm to stabilize the patient until they are brought to a hospital for treatment. While it may be lifesaving, the aortic occlusion, can cause severe long term consequences for patients. This project focuses on developing a novel device to enable controllable occlusion of the aorta to allow stabilization of the hemorrhage without completely cutting off blood flow to the lower extremities. The device will be designed to provide a safer alternative to current devices for management of non-compressible hemorrhage. The device will be designed to be easy to use by enabling automation of the hemorrhage control process via robotic technologies.
Meet the Team
 Dr. Tom Ranzani received a Bachelor’s and Master’s degree in Biomedical Engineering from the University of Pisa, Italy. He did his Ph.D. at the BioRobotics Institute of the Sant’Anna School of Advanced Studies. In 2014, he joined the Wyss Institute for Biologically Inspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences as a postdoctoral fellow.
Dr. Tom Ranzani received a Bachelor’s and Master’s degree in Biomedical Engineering from the University of Pisa, Italy. He did his Ph.D. at the BioRobotics Institute of the Sant’Anna School of Advanced Studies. In 2014, he joined the Wyss Institute for Biologically Inspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences as a postdoctoral fellow.
He is currently an Assistant Professor in the Department of Mechanical Engineering, Biomedical Engineering, and in the Division of Materials Science and Engineering at Boston University, where he established the Morphable Biorobotics Lab in 2018.
In 2020 he was awarded the NIH Trailblazer Award for New and Early Stage Investigators.
His research focuses on soft and bioinspired robotics with applications ranging from underwater exploration to surgical and wearable devices. He is interested in expanding the potential of soft robots across different scales to develop novel reconfigurable soft-bodied robots capable of operating in environments where traditional robots cannot.
 Dr. Jeffrey Siracuse, is Professor of Surgery and Radiology at the Bostin University Chobanian & Avedisian School of Medicine and Associate Chair of Surgery for Quality and Patient Safety at Boston Medical Center. Dr. Siracuse completed general surgery residency at Beth Israel Deaconess Medical Center and a fellowship in Vascular and Endovascular Surgery at New York-Presbyterian Hospital. He was recruited to Boston Medical Center (BMC)/BU Chobanian & Avedisian School of Medicine in 2014. Since 2018, he has served as the Program Director of the BMC Vascular and Endovascular Surgery Fellowship two years later. Dr. Siracuse has been the principal investigator on a large number of research projects, with external support from the NIH and industry. He was the founding Chair of the Society for Vascular Surgery’s Appropriateness Committee and led the development of Appropriateness Use Criteria for the treatment of patients with claudication. As the Medical Director of the Vascular Study Group of New England, which focuses on improving quality and safety in caring for patients with vascular disease, he oversees quality initiatives and the awarding of grants. Dr. Siracuse is the author of more than 250 peer-reviewed journal articles. He has held leadership positions in national vascular surgery organizations and has co-authored multiple practice guidelines. He is an editor of Annals of Vascular Surgery and serves on the editorial board of the Journal of Vascular Surgery.
Dr. Jeffrey Siracuse, is Professor of Surgery and Radiology at the Bostin University Chobanian & Avedisian School of Medicine and Associate Chair of Surgery for Quality and Patient Safety at Boston Medical Center. Dr. Siracuse completed general surgery residency at Beth Israel Deaconess Medical Center and a fellowship in Vascular and Endovascular Surgery at New York-Presbyterian Hospital. He was recruited to Boston Medical Center (BMC)/BU Chobanian & Avedisian School of Medicine in 2014. Since 2018, he has served as the Program Director of the BMC Vascular and Endovascular Surgery Fellowship two years later. Dr. Siracuse has been the principal investigator on a large number of research projects, with external support from the NIH and industry. He was the founding Chair of the Society for Vascular Surgery’s Appropriateness Committee and led the development of Appropriateness Use Criteria for the treatment of patients with claudication. As the Medical Director of the Vascular Study Group of New England, which focuses on improving quality and safety in caring for patients with vascular disease, he oversees quality initiatives and the awarding of grants. Dr. Siracuse is the author of more than 250 peer-reviewed journal articles. He has held leadership positions in national vascular surgery organizations and has co-authored multiple practice guidelines. He is an editor of Annals of Vascular Surgery and serves on the editorial board of the Journal of Vascular Surgery.
Funded by BU CTSI CE |
Developing a Family-Based Program to Enhance Autonomy in Adults with Down Syndrome
做
Meet the Team
 Dr. Gael Osmond is a Professor in the Department of Occupational Therapy and Associate Dean of Academic Affairs at the Sargent College of Health & Rehabilitation Sciences. Her research, funded by NIMH, NIA, NICHD, the Institute of Education Sciences, and the Deborah Munroe Noonan Memorial Research Fund, focuses primarily on the transition to adulthood in individuals with developmental disabilities, including autism, and how the family, social, community, and school contexts support the wellbeing of adolescents and adults with developmental disabilities. Dr. Orsmond has published more than 65 peer-reviewed publications and book chapters. She is a fellow of Division 33 (intellectual and developmental disabilities/autism) of the American Psychological Association.
Dr. Gael Osmond is a Professor in the Department of Occupational Therapy and Associate Dean of Academic Affairs at the Sargent College of Health & Rehabilitation Sciences. Her research, funded by NIMH, NIA, NICHD, the Institute of Education Sciences, and the Deborah Munroe Noonan Memorial Research Fund, focuses primarily on the transition to adulthood in individuals with developmental disabilities, including autism, and how the family, social, community, and school contexts support the wellbeing of adolescents and adults with developmental disabilities. Dr. Orsmond has published more than 65 peer-reviewed publications and book chapters. She is a fellow of Division 33 (intellectual and developmental disabilities/autism) of the American Psychological Association.
 Dr. Kristin Long is an Associate Professor in the Department of Psychological and Brain Sciences. Her research investigates individuals’ and families’ experiences of medical illness and disability across the life course in an effort to develop interventions that promote psychosocial functioning within family-centered, culturally-informed models of care. Dr. Long has formed meaningful community partnerships to increase the relevance and community impact of her work. She has led efforts to form local, national, and international collaborations to better understand how to address pervasive unmet needs among individuals with chronic medical conditions or disability and their families. Dr. Long has received funding from the National Institutes of Health and numerous foundations to support her work.
Dr. Kristin Long is an Associate Professor in the Department of Psychological and Brain Sciences. Her research investigates individuals’ and families’ experiences of medical illness and disability across the life course in an effort to develop interventions that promote psychosocial functioning within family-centered, culturally-informed models of care. Dr. Long has formed meaningful community partnerships to increase the relevance and community impact of her work. She has led efforts to form local, national, and international collaborations to better understand how to address pervasive unmet needs among individuals with chronic medical conditions or disability and their families. Dr. Long has received funding from the National Institutes of Health and numerous foundations to support her work.
Co-funded by BU CTSI & GSDM |
Radiation Therapy Resistance Promoted by LSD1 in Oral Cancer
Radiation therapy resistance promoted by LSD1 in oral cancer,”. Radiation therapy (RT) has been extensively used for locally advanced cancer. The study showed that only 20-30% of tumors respond to RT. Thus, there is an unmet clinical need to identify radiosensitizers. We have identified an epigenetic regulator, Lysine-specific demethylase 1 (LSD1), which promotes oral and resistance to RT. We expect to evaluate the novel mechanisms that LSD1 promotes DNA damage repair response signaling pathways and inhibition, which could promote sensitivity to RT using a mouse model and pathway analysis. Dr. Truong, who is clinical radiation oncologist and expert in radiation therapy who will provide help in evaluating the doses and relevance of findings for future translation.
Meet the Team
 Dr. Manish Bais’s lab focuses on oral cancer and skeletal biology, including temporomandibular joint (TMJ) degeneration and osteoarthritis. He is also a veterinarian by training, specializes in molecular and genetic studies of oral cancer and bone and cartilage remodeling, and develops novel mouse models for translating basic science findings to preclinical studies. He has shared preclinical models with investigators at Boston University and with the wider scientific community.
Dr. Manish Bais’s lab focuses on oral cancer and skeletal biology, including temporomandibular joint (TMJ) degeneration and osteoarthritis. He is also a veterinarian by training, specializes in molecular and genetic studies of oral cancer and bone and cartilage remodeling, and develops novel mouse models for translating basic science findings to preclinical studies. He has shared preclinical models with investigators at Boston University and with the wider scientific community.
She has published over 100 peer-reviewed scientific articles, editorials, book chapters, and invited reviews.
In 2022, Dr Truong was awarded the Fellow of the American Society of Radiation Oncology for her contributions to the field of Radiation Oncology and service to ASTRO.
Since 2017 to 2024, she has been named Top Doctor in Castle Connolly’s America’s Top Doctors, Exceptional Women in Medicine, and Boston Magazine.
Funded by DOM |
Dialysis Modality Education Access for Latinx Individuals with Kidney Disease
Latinx people experience a two times greater risk of kidney failure compared to non-Latinx White individuals yet are less likely to receive adequate preparation for kidney failure- leading to high rates of urgent dialysis initiation and lower rates of home dialysis and transplant. Pre-dialysis kidney disease education is associated with significantly higher odds of patient-centered preparation for dialysis amongst Latinx individuals. However, Latinx people are less likely to receive adequate kidney dialysis education prior to dialysis, and are more likely to report lack of shared decision-making with their clinician. The aim of our study is to 1) qualitatively evaluate the dialysis modality education experience for Latinx people with kidney disease and their clinicians and 2) establish a community research steering committee to co-design culturally tailored dialysis modality educational materials for Latinx individuals with kidney disease.
 Dr. Katherine “Katie” Rizzolo我
Dr. Katherine “Katie” Rizzolo我
In-depth Profiling of Sickle Cell Disease (SCD) Hematopoietic Stem and Progenitor Cells (HSPCs) to Advance Gene Therapy and Transplantation Approaches
Th
Meet the Team
 Dr. Kim Vanuytsel is a stem cell biologist with expertise in developmental hematopoiesis, sickle cell disease and hematopoietic stem cell biology. She is originally from Belgium and obtained her PhD from KULeuven (Leuven, Belgium). She subsequently joined the laboratory of George Murphy at the Center for Regenerative Medicine (CReM) for her postdoctoral work. Since joining the research community at Boston University and Boston Medical Center, she has focused on developing tools and resources that help us understand important concepts in hematopoietic development with the goal of translating this knowledge into the realization of the immense potential that induced pluripotent stem cells (iPSCs) and hematopoietic stem cells hold for disease modeling and regenerative medicine.
Dr. Kim Vanuytsel is a stem cell biologist with expertise in developmental hematopoiesis, sickle cell disease and hematopoietic stem cell biology. She is originally from Belgium and obtained her PhD from KULeuven (Leuven, Belgium). She subsequently joined the laboratory of George Murphy at the Center for Regenerative Medicine (CReM) for her postdoctoral work. Since joining the research community at Boston University and Boston Medical Center, she has focused on developing tools and resources that help us understand important concepts in hematopoietic development with the goal of translating this knowledge into the realization of the immense potential that induced pluripotent stem cells (iPSCs) and hematopoietic stem cells hold for disease modeling and regenerative medicine.
As part of the Center of Excellence in Sickle Cell Disease here at Boston Medical Center, serving a large and diverse sickle cell disease patient population, Dr. Vanuytsel is committed to finding better solutions for these patients. Leading her research lab, embedded within the CReM, her goal is to continue to focus on issues at the intersection of stem cell biology, cell therapies and sickle cell disease. Her experience in these diverse but complimentary research fields has equipped Dr. Vanuytsel with a unique perspective and skillset to make meaningful contributions to emerging cell therapies for sickle cell disease patients, and by extension, the field of hematopoietic stem cell transplantation as a whole.
 Dr. Jean-Antoine Ribeil, is a French clinical hematologist recognized for his dedication to advancing research in red cell disorders, particularly hemoglobinopathies. With a focus on translational research, he spent over a decade at Necker University Hospital in Paris, leading research programs on erythropoiesis and red blood cell disorders while providing care to adult patients with hemoglobin disorders.
Dr. Jean-Antoine Ribeil, is a French clinical hematologist recognized for his dedication to advancing research in red cell disorders, particularly hemoglobinopathies. With a focus on translational research, he spent over a decade at Necker University Hospital in Paris, leading research programs on erythropoiesis and red blood cell disorders while providing care to adult patients with hemoglobin disorders.
Dr. Ribeil’s work has contributed to the development and implementation of gene therapy protocols for hemoglobinopathies, including the recent FDA approvals of Lentiglobin gene therapy for thalassemia and sickle cell disease (SCD). His investigations have also played a role in hematopoietic stem cell collection studies for gene therapy, including a pioneering study on hematopoietic stem cell collection for sickle cell disease using only plerixafor.
Since May 2021, Dr. Ribeil has served as the Clinical Director of the Sickle Cell Center for Excellence at Boston University Medical Center, where his main aim is to provide exceptional care to improve the quality of life for patients. He also leads the GLOB research group, dedicated to advancing research on hemoglobinopathies and gene therapy with the ultimate goal of making these programs accessible to patients, and improving patient outcomes.
Cardiopulmonary Biomarkers of Systemic Sclerosis
Pulmonary hypertension is a major manifestation of a connective tissue disease known as systemic sclerosis and is a significant cause of mortality for many of these patients. Systemic sclerosis-related pulmonary hypertension is a heterogeneous disease with multifactorial contributions. In this proposal, we investigate the role of cardiac disease using an advanced imaging approach, termed speckle-tracking echocardiography, that measures subclinical myocardial deformation to identify and create a library of associated protein biomarkers in the blood. The goal is to unravel potential mechanistic pathways to explain cardiac contributors to pulmonary hypertension for determining why these patients have such poor clinical outcomes.
Meet the Team
 Dr. Justin K. Lui, is a pulmonary and critical care physician and an Assistant Professor of Medicine in the Section of Pulmonary, Allergy, Sleep & Critical Care Medicine. Dr. Lui’s clinical and research interest is in pulmonary hypertension, specifically in systemic sclerosis. Within the Pulmonary Center, he conducts clinical and translational research that is at the intersection of medicine and engineering in applying advanced imaging and data science methodologies for a deep cardiopulmonary phenotyping of this rare disease population to further advance novel diagnostic and therapeutic approaches.
Dr. Justin K. Lui, is a pulmonary and critical care physician and an Assistant Professor of Medicine in the Section of Pulmonary, Allergy, Sleep & Critical Care Medicine. Dr. Lui’s clinical and research interest is in pulmonary hypertension, specifically in systemic sclerosis. Within the Pulmonary Center, he conducts clinical and translational research that is at the intersection of medicine and engineering in applying advanced imaging and data science methodologies for a deep cardiopulmonary phenotyping of this rare disease population to further advance novel diagnostic and therapeutic approaches.
 Dr. Andreea M. Bujor, is an Associate Professor of Medicine in the Section of Rheumatology at Boston University Chobanian & Avedisian School of Medicine, and the Associate Program Director for the Rheumatology Fellowship Program. She is a board-certified rheumatologist and physician-scientist who specializes in the care of patients with systemic sclerosis. Dr. Bujor has a research lab within the Arthritis and Autoimmune Diseases Center where she conducts translational research in systemic sclerosis. Dr. Bujor’s research focuses on the interaction between immunity and fibrosis in systemic sclerosis, identification of new biomarkers of disease, and development and validation of novel outcome measures for clinical trials in scleroderma.
Dr. Andreea M. Bujor, is an Associate Professor of Medicine in the Section of Rheumatology at Boston University Chobanian & Avedisian School of Medicine, and the Associate Program Director for the Rheumatology Fellowship Program. She is a board-certified rheumatologist and physician-scientist who specializes in the care of patients with systemic sclerosis. Dr. Bujor has a research lab within the Arthritis and Autoimmune Diseases Center where she conducts translational research in systemic sclerosis. Dr. Bujor’s research focuses on the interaction between immunity and fibrosis in systemic sclerosis, identification of new biomarkers of disease, and development and validation of novel outcome measures for clinical trials in scleroderma.
 Dr. Michael P. LaValley is a Professor of Biostatistics at Boston University School of Public Health, where he teaches courses on meta-analysis, logistic regression, and survival analysis. Dr. LaValley serves as the Research Director of the Boston University Core Center for Clinical Research, is the co-lead of the Analysis Core for the Multicenter Osteoarthritis Study (MOST) and is a Member of the Arthritis and Musculoskeletal and Skin Diseases Clinical Trials Study Section (AMSC) for the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Dr. Michael P. LaValley is a Professor of Biostatistics at Boston University School of Public Health, where he teaches courses on meta-analysis, logistic regression, and survival analysis. Dr. LaValley serves as the Research Director of the Boston University Core Center for Clinical Research, is the co-lead of the Analysis Core for the Multicenter Osteoarthritis Study (MOST) and is a Member of the Arthritis and Musculoskeletal and Skin Diseases Clinical Trials Study Section (AMSC) for the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
2023 Integrated Pilot Grant Awardees
Co-funded by BMC & BU CTSI |
Naltrexone for the Treatment of Cannabis Use Disorder in Pregnancy
Th
The primary outcome measure of this study will be quantity of cannabis use, defined by survey and laboratory data assessing cannabis consumption. All key outcome measures are as follows: 1) participant outcomes [cannabis consumption, retention in care, preterm birth, and side effects / adverse events); 2) fetal outcomes (intrauterine growth, fetal anomalies).
Meet the Team
Co-funded by BMC & DOM |
Assessing and Addressing Implicit Bias within Healthcare-acquired Infections (AAIm HI)
圣
Meet the Team
Dr. Shana A. B. Burrowes is an Assistant Professor at Boston University Chobanian & Avedisian School of Medicine. Dr. Burrowes is a trained epidemiologist skilled in advanced statistical analysis techniques, which she applies across a diverse set of content areas including antibiotic stewardship, HIV, cardiovascular disease, Hepatitis C, sickle cell anemia, and COVID-19. She has a particular interest in applying her analytical and study design skills to projects of public health importance, with a focus on addressing healthcare inequities in underrepresented populations.
 Dr. Cassandra M. Pierre is an Assistant Professor at Boston University’s School of Medicine, the Medical Director of Public Health Programs, the Associate Hospital Epidemiologist at Boston Medical Center, and a member of Boston University’s Center for Emerging Infectious Diseases. Her research is focused on infection prevention in systemically vulnerable populations and the elimination of race-based infectious disease inequities.
Dr. Cassandra M. Pierre is an Assistant Professor at Boston University’s School of Medicine, the Medical Director of Public Health Programs, the Associate Hospital Epidemiologist at Boston Medical Center, and a member of Boston University’s Center for Emerging Infectious Diseases. Her research is focused on infection prevention in systemically vulnerable populations and the elimination of race-based infectious disease inequities.
 Dr. Angelique C. Harris is the Associate Dean for Diversity & Inclusion at the Chobanian & Avedisian School of Medicine, the Executive Director of Faculty Development for BU Medical Campus, and Associate Professor in the Department of Medicine and in the Section, General Internal Medicine. Dr. Harris creates, implements, and leads innovative programs, trainings, and initiatives designed to promote more inclusive working and learning environments for faculty, students, and staff in the academic health sciences and STEMM. An Applied Medical Sociologist, Dr. Harris’s other areas of research include health and community activism, race and ethnicity, gender and sexualities, religious studies, cultural studies, and has conducted extensive research exploring sociocultural constructions of health, illness, and medicine within structurally marginalized communities.
Dr. Angelique C. Harris is the Associate Dean for Diversity & Inclusion at the Chobanian & Avedisian School of Medicine, the Executive Director of Faculty Development for BU Medical Campus, and Associate Professor in the Department of Medicine and in the Section, General Internal Medicine. Dr. Harris creates, implements, and leads innovative programs, trainings, and initiatives designed to promote more inclusive working and learning environments for faculty, students, and staff in the academic health sciences and STEMM. An Applied Medical Sociologist, Dr. Harris’s other areas of research include health and community activism, race and ethnicity, gender and sexualities, religious studies, cultural studies, and has conducted extensive research exploring sociocultural constructions of health, illness, and medicine within structurally marginalized communities.
Funded by BU CTSI |
Association between Hip Shape and Hip Symptoms
操作系统
Meet the Team
 Dr. Cara L. Lewis is an Associate Professor in the Department of Physical Therapy within Boston University’s College of Health and Rehabilitation Sciences: Sargent College. She received her Master of Science in Physical Therapy from Washington University in St. Louis. She practiced physical therapy for 4 years before returning to Washington University for her PhD in Movement Science. Dr. Lewis completed a post-doctoral fellowship focused on rehabilitation robotics with Dan Ferris, PhD, at the University of Michigan in Ann Arbor. Dr. Lewis has published several peer-reviewed journal articles on hip joint forces, movement analysis and gait. She has received research funding from multiple sources included the NIH and NSF. Dr. Lewis is currently funded to investigate movement differences in young adults with and without hip pain.
Dr. Cara L. Lewis is an Associate Professor in the Department of Physical Therapy within Boston University’s College of Health and Rehabilitation Sciences: Sargent College. She received her Master of Science in Physical Therapy from Washington University in St. Louis. She practiced physical therapy for 4 years before returning to Washington University for her PhD in Movement Science. Dr. Lewis completed a post-doctoral fellowship focused on rehabilitation robotics with Dan Ferris, PhD, at the University of Michigan in Ann Arbor. Dr. Lewis has published several peer-reviewed journal articles on hip joint forces, movement analysis and gait. She has received research funding from multiple sources included the NIH and NSF. Dr. Lewis is currently funded to investigate movement differences in young adults with and without hip pain.
 Dr. Elise Morgan is the Maysarah K. Sukkar Professor of Engineering Design and Innovation and the inaugural Director of the Center for Multiscale and Translational Mechanobiology at Boston University. 上海Journal of Biomechanics. She is the recipient of several research awards, including the 2013 Kappa Delta Young Investigator Award from the American Academy of Orthopaedic Surgeons, and is the co-founder of a STEM outreach program, Summer Pathways, for high-school girls. Dr. Morgan currently serves as the Associate Dean for Research and Faculty Development in the College of Engineering. She serves on the editorial board of Bone and is a member of the College of Fellows of the American Institute for Medical and Biological Engineering.
Dr. Elise Morgan is the Maysarah K. Sukkar Professor of Engineering Design and Innovation and the inaugural Director of the Center for Multiscale and Translational Mechanobiology at Boston University. 上海Journal of Biomechanics. She is the recipient of several research awards, including the 2013 Kappa Delta Young Investigator Award from the American Academy of Orthopaedic Surgeons, and is the co-founder of a STEM outreach program, Summer Pathways, for high-school girls. Dr. Morgan currently serves as the Associate Dean for Research and Faculty Development in the College of Engineering. She serves on the editorial board of Bone and is a member of the College of Fellows of the American Institute for Medical and Biological Engineering.
Determining Contributions of Hepatocyte Heterogeneity to ZZ AATD-Associated Liver Disease
艾尔
Meet the Team
Individual and Neighborhood-Level Disparities in Buprenorphine Treatment Among BMC Patients
人事处
Meet the Team
 Dr. Alyssa Tilhou is a family physician, addiction specialist and health services researcher in the Department of Family Medicine at Boston Medical Center. She is also the Assistant Research Director in the Department of Family Medicine. Her research focuses on access and utilization of primary care and substance use services in low-income populations. Dr. Tilhou completed her medical education and doctorate in Population Health Sciences at the University of Texas Medical Branch, residency in family medicine at the Mountain Area Health Education Center in Asheville, NC, and a fellowship in Addiction Medicine at the University of Wisconsin. She is supported by a K08 from the National Institute on Drug Abuse.
Dr. Alyssa Tilhou is a family physician, addiction specialist and health services researcher in the Department of Family Medicine at Boston Medical Center. She is also the Assistant Research Director in the Department of Family Medicine. Her research focuses on access and utilization of primary care and substance use services in low-income populations. Dr. Tilhou completed her medical education and doctorate in Population Health Sciences at the University of Texas Medical Branch, residency in family medicine at the Mountain Area Health Education Center in Asheville, NC, and a fellowship in Addiction Medicine at the University of Wisconsin. She is supported by a K08 from the National Institute on Drug Abuse.
 Dr. William G. Adams is an epidemiologist, medical informatician, and practicing pediatrician at Boston Medical Center (BMC). He is also Professor of Pediatrics and serves as Director of BU-CTSI Biomedical Informatics for Boston University and Director of Community Health Informatics for the Boston HealthNet – an urban integrated health delivery system. His primary research is focused on developing and evaluating information technology (IT)-based solutions for improving the quality of health and healthcare for urban populations. His foci include electronic health records (EHR) for research, state-wide registries, decision support, patient-centered health IT and clinical data warehousing for quality improvement and comparative effectiveness research.
Dr. William G. Adams is an epidemiologist, medical informatician, and practicing pediatrician at Boston Medical Center (BMC). He is also Professor of Pediatrics and serves as Director of BU-CTSI Biomedical Informatics for Boston University and Director of Community Health Informatics for the Boston HealthNet – an urban integrated health delivery system. His primary research is focused on developing and evaluating information technology (IT)-based solutions for improving the quality of health and healthcare for urban populations. His foci include electronic health records (EHR) for research, state-wide registries, decision support, patient-centered health IT and clinical data warehousing for quality improvement and comparative effectiveness research.
 Dr. Katherine R. Standish is a clinician and researcher in the Department of Family Medicine at Boston University and is co-founder of the Breastfeeding Equity Center at BMC. Her research addresses breastfeeding services and interventions in marginalized and high-risk populations including dyads impacted by maternal substance use. Dr. Standish studied medicine at Yale University, completed residency training and a primary care academic fellowship at Boston Medical Center, and earned an MS in Epidemiology at Boston University School of Public Health. Prior to studying medicine, she worked in epidemiologic and community-based participatory research on opioid use disorder and infectious diseases in the U.S., Mexico, and Nicaragua.
Dr. Katherine R. Standish is a clinician and researcher in the Department of Family Medicine at Boston University and is co-founder of the Breastfeeding Equity Center at BMC. Her research addresses breastfeeding services and interventions in marginalized and high-risk populations including dyads impacted by maternal substance use. Dr. Standish studied medicine at Yale University, completed residency training and a primary care academic fellowship at Boston Medical Center, and earned an MS in Epidemiology at Boston University School of Public Health. Prior to studying medicine, she worked in epidemiologic and community-based participatory research on opioid use disorder and infectious diseases in the U.S., Mexico, and Nicaragua.
博士
Pilot Feasibility Study of an In-Home, Body Weight Harness Mobility System for Infants with Down syndrome
The emergence of crawling and walking is significantly delayed in infants with Down syndrome (DS), but the development of independent mobility provides infants with new opportunities for exploring the environment and interacting with objects and people, which are important foundations for early learning. Increasing infant mobility early in development with body weight supported harness systems may support infant exploration, communication, and social interaction. This project will set the stage for the first clinical trial of a mobility-related intervention specifically tailored for infants with DS by testing the feasibility of harness systems with infants and families and identifying measures that will serve as primary outcome variables. Upon completion of this pilot project, Dr. Jana Iverson and Dr. Nicole Baumer will have obtained necessary preliminary data and experience required for an in-home, high-impact clinical trial for infants with DS.
Meet the Team
 Dr. Jana M. Iverson is the Dudley Allen Sargent Professor of Pediatric Rehabilitation and Associate Dean for Research for the College of Health & Rehabilitation Sciences: Sargent College at Boston University. Her research, funded by NICHD, NIDCD, and Autism Speaks, focuses primarily on the interface between the development of early motor skills and the emergence of communication and language in neurotypical development and in children with or at risk for developmental disorders. Dr. Iverson has published a co-edited book and more than 100 articles and book chapters. She is on the editorial boards of the Journal of Child Language and Language Learning and Development. Since 1991, she has served as an international investigator at the CNR in Rome, Italy. Dr. Iverson is a Fellow of the Association for Psychological Science.
Dr. Jana M. Iverson is the Dudley Allen Sargent Professor of Pediatric Rehabilitation and Associate Dean for Research for the College of Health & Rehabilitation Sciences: Sargent College at Boston University. Her research, funded by NICHD, NIDCD, and Autism Speaks, focuses primarily on the interface between the development of early motor skills and the emergence of communication and language in neurotypical development and in children with or at risk for developmental disorders. Dr. Iverson has published a co-edited book and more than 100 articles and book chapters. She is on the editorial boards of the Journal of Child Language and Language Learning and Development. Since 1991, she has served as an international investigator at the CNR in Rome, Italy. Dr. Iverson is a Fellow of the Association for Psychological Science.
 Dr. Nicole Baumer is a child neurologist / neurodevelopmental disabilities specialist at Boston Children’s Hospital and an Assistant Professor of Neurology at Harvard Medical School. Dr. Baumer is Director of the Boston Children’s Hospital Down Syndrome Program. She completed medical training at Harvard Medical school, pediatrics training at Massachusetts General Hospital, and Neurodevelopmental Disabilities Training at Boston Children’s Hospital. Dr. Baumer also studied Special Education, and has a Masters Degree in Education from Harvard Graduate School of Education. She specializes in treatment of individuals with Down syndrome, Autism, ADHD, and other neurobehavioral disorders. Dr. Baumer’s research involves characterization and diagnosis of neurodevelopmental profiles in Down syndrome, and investigation of educational, behavioral, and medical interventions in neurodevelopmental disorders.
Dr. Nicole Baumer is a child neurologist / neurodevelopmental disabilities specialist at Boston Children’s Hospital and an Assistant Professor of Neurology at Harvard Medical School. Dr. Baumer is Director of the Boston Children’s Hospital Down Syndrome Program. She completed medical training at Harvard Medical school, pediatrics training at Massachusetts General Hospital, and Neurodevelopmental Disabilities Training at Boston Children’s Hospital. Dr. Baumer also studied Special Education, and has a Masters Degree in Education from Harvard Graduate School of Education. She specializes in treatment of individuals with Down syndrome, Autism, ADHD, and other neurobehavioral disorders. Dr. Baumer’s research involves characterization and diagnosis of neurodevelopmental profiles in Down syndrome, and investigation of educational, behavioral, and medical interventions in neurodevelopmental disorders.
Risk Assessment of Lung Squamous Premalignant Lesions
陆
Meet the Team
 Dr. Jennifer E. Beane-Ebel is an Associate Professor of Medicine in the Section of Computational Biomedicine within the Department of Medicine at BUSM. She is a computational biologist with research interests in developing early detection lung cancer biomarkers and understanding biological mechanism of early lung cancer initiation and development.
Dr. Jennifer E. Beane-Ebel is an Associate Professor of Medicine in the Section of Computational Biomedicine within the Department of Medicine at BUSM. She is a computational biologist with research interests in developing early detection lung cancer biomarkers and understanding biological mechanism of early lung cancer initiation and development.
 Dr. Vijaya B. Kolachalama is an Associate Professor of Medicine in the Section of Computational Biomedicine within the Department of Medicine at BUSM. He is a founding member of BU’s Faculty of Computing & Data Sciences and affiliated with the Department of Computer Science. Research interests of his group currently lie at the interface of machine vision, representation learning and domain generalization.
Dr. Vijaya B. Kolachalama is an Associate Professor of Medicine in the Section of Computational Biomedicine within the Department of Medicine at BUSM. He is a founding member of BU’s Faculty of Computing & Data Sciences and affiliated with the Department of Computer Science. Research interests of his group currently lie at the interface of machine vision, representation learning and domain generalization.
Other Members of the Team
Dr. Eric Burks, Dr. Sarah Mazzilli, & Dr. Ehab Billatos
Target Deconvolution Of Host-Directed Antiviral Rocaglates
The continued spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) warrants discovery of new antiviral therapeutics. To address this need, a team led by Prof. John Porco (PI, BU Chemistry BU-CMD and co-Investigator Prof. Robert Davey (BU-NEIDL) employed a cell-based screen that identified a number of synthetic rocaglates as potent inhibitors of SARS-CoV-2 infection. Rocaglates are a diverse family of plant metabolites with potent anti-neoplastic and anti-infective activities. In the CTSI Integrated Pilot Grant “Target Deconvolution of Host-Antiviral Rocaglates,” a two-phased approach will be used to identify rocaglate host targets that are important for SARS-CoV-2 infection. The approach involves use of a novel thermal proteome profiling assay to identify candidate targets of antiviral rocaglates, followed by a targeted CRISPR screen to disrupt candidate targets and evaluate impact on infection efficiency.
Meet the Team
 Dr John A. Porco joined the Department of Chemistry at Boston University in 1999 as Assistant Professor after working in industry and was promoted to Professor of Chemistry in September 2004. Since 2014, John has been the Director of the Center for Molecular Discovery (BU), an integrated infrastructure for the discovery of small molecule chemical probes and medicinal chemistry. The Porco Laboratory develops new synthetic methodologies for chemical synthesis of bioactive molecules and complex natural products. The laboratory also seeks to establish innovation in collaborative, translational science to study the biological properties and mode of action (MoA) of target molecules including collaborative work with the Davey lab. The Porco lab is interfaced with the nearby Boston University Center for Molecular Discovery (BU-CMD) and the newly establish BU Target Discovery Lab (BU-TDL).
Dr John A. Porco joined the Department of Chemistry at Boston University in 1999 as Assistant Professor after working in industry and was promoted to Professor of Chemistry in September 2004. Since 2014, John has been the Director of the Center for Molecular Discovery (BU), an integrated infrastructure for the discovery of small molecule chemical probes and medicinal chemistry. The Porco Laboratory develops new synthetic methodologies for chemical synthesis of bioactive molecules and complex natural products. The laboratory also seeks to establish innovation in collaborative, translational science to study the biological properties and mode of action (MoA) of target molecules including collaborative work with the Davey lab. The Porco lab is interfaced with the nearby Boston University Center for Molecular Discovery (BU-CMD) and the newly establish BU Target Discovery Lab (BU-TDL).
Co-funded by BU CTSI & DOM |
Big Data and Team Science Approach to Identify Novel Molecular Pathway and Mechanism of Action of a New Drug for Patients with Chronic Kidney Disease
Chronic kidney disease (CKD) affects approximately 13% of the global population, yet there is currently no kidney podocyte-specific anti-proteinuric treatment available, highlighting a significant global medical need. This pilot project aims to address this gap by identifying a novel molecular pathway and mechanism of action for a potential drug to treat proteinuria and CKD, using a team science approach. The project will leverage pathological and single-cell transcriptome data from animal models to gain a deeper understanding of the underlying mechanisms of potential candidate drugs. Furthermore, it will validate and translate these findings using CKD patient data and samples collected as part of the KPMP and BKBC studies. Successful completion of this project has the potential to yield new therapeutics targeting novel drug targets for CKD and proteinuria.
Meet the Team
 Dr. Chao Zhang is an Assistant Professor of Computational Biomedicine in the Department of Medicine at Boston University School of Medicine. As an interdisciplinary researcher, he has a background in systems biology, statistics, machine learning, software engineering, and especially in next-generation sequencing analysis. His current research is centered around the investigation of aging-related and chronic diseases, including chronic kidney disease, Alzheimer’s disease, and degenerative joint diseases. Through the use of computational methods, Dr. Zhang aims to develop innovative deep-learning approaches to analyze the single cell level molecular data, providing valuable insights into disease mechanisms. Furthermore, he integrates clinical data with molecular data to enhance disease diagnosis and treatment strategies.
Dr. Chao Zhang is an Assistant Professor of Computational Biomedicine in the Department of Medicine at Boston University School of Medicine. As an interdisciplinary researcher, he has a background in systems biology, statistics, machine learning, software engineering, and especially in next-generation sequencing analysis. His current research is centered around the investigation of aging-related and chronic diseases, including chronic kidney disease, Alzheimer’s disease, and degenerative joint diseases. Through the use of computational methods, Dr. Zhang aims to develop innovative deep-learning approaches to analyze the single cell level molecular data, providing valuable insights into disease mechanisms. Furthermore, he integrates clinical data with molecular data to enhance disease diagnosis and treatment strategies.
 Dr. Sudhir Kumar is an Assistant Professor of Medicine in the Nephrology Section, Department of Medicine at Boston University School of Medicine. His research work focuses on Slit-Robo signaling in kidney diseases and podocyte biology and ZEB2 signaling in kidney development and diseases using animal models. Dr. Kumar received his Ph.D. from Ludwig Maximilians University Munich, Germany and completed his postdoctoral training in Dr. Weining Lu’s lab, Nephrology Section at Boston University School of Medicine.
Dr. Sudhir Kumar is an Assistant Professor of Medicine in the Nephrology Section, Department of Medicine at Boston University School of Medicine. His research work focuses on Slit-Robo signaling in kidney diseases and podocyte biology and ZEB2 signaling in kidney development and diseases using animal models. Dr. Kumar received his Ph.D. from Ludwig Maximilians University Munich, Germany and completed his postdoctoral training in Dr. Weining Lu’s lab, Nephrology Section at Boston University School of Medicine.
Funded by DOM |
Benchmarking Intra-tumor Heterogeneity Approaches in Aggressive Breast Cancer
来
Meet the Team
博士
博士
Defining a Molecular Signature of Cardiac Dysfunction in Systemic Immunoglobulin Light Chain Amyloidosis
Systemic immunoglobulin light chain (AL) amyloidosis features misfolding of destabilized light chains (LCs) produced by malignant plasma cells in bone marrow. Misfolded LCs become amyloid fibrils that deposit in target organs. Organ dysfunction leads to poorer prognosis of AL amyloidosis than other plasma cell dyscrasias. Specifically, advanced cardiac dysfunction limits treatment options and causes early mortality. Cardiac dysfunction is directly precipitated by: 1) direct effects of destabilized LCs and eventually 2) structural damage from amyloid fibril deposits. Understanding early mechanisms of cardiac dysfunction is key to developing screening methods and treatments critical to improving these outcomes. Therefore, the team seeks to identify and validate a molecular signature for early mechanisms of LC-mediated cardiac dysfunction using patient-derived LCs in a novel disease model of induced pluripotent stem cells.
The Relationship of Intra-articular Mineralization to Synovitis in Knee Osteoarthritis
Knee osteoarthritis (OA) affects a large number of adults in the United States and is a major cause of disability. Current treatments for Knee OA are limited in their ability to prevent disease progression and alleviate symptoms. The failure of OA therapies to date is partly due to not targeting the appropriate at-risk populations. Focusing on a Knee OA subtype, those with intra-articular (IA) mineralization, may lead to more effective treatment through the development of targeted mechanistic approaches. This proposal aims to investigate the relationship between IA mineralization and inflammation (synovitis on MRI) in Knee OA. The study will provide valuable insights into how IA mineralization contributes to inflammation, potentially leading to improved therapies, such as anti-inflammatory treatments, for specific groups of people with Knee OA.
 Dr. Jean W. Liew obtained her MD from the University of Texas Medical Branch in Galveston, TX. Following this, she completed her Internal Medicine residency at Oregon Health & Science University in Portland, OR and a rheumatology fellowship at the University of Washington in Seattle, WA. Alongside her fellowship, she earned an M.S. in Epidemiology through the University of Washington School of Public Health. Presently, Dr. Liew serves as an Assistant Professor of Medicine in the Section of Rheumatology at BU. Her clinical research centers around knee osteoarthritis and axSpA.
Dr. Jean W. Liew obtained her MD from the University of Texas Medical Branch in Galveston, TX. Following this, she completed her Internal Medicine residency at Oregon Health & Science University in Portland, OR and a rheumatology fellowship at the University of Washington in Seattle, WA. Alongside her fellowship, she earned an M.S. in Epidemiology through the University of Washington School of Public Health. Presently, Dr. Liew serves as an Assistant Professor of Medicine in the Section of Rheumatology at BU. Her clinical research centers around knee osteoarthritis and axSpA.
Funded by BU CTSI Informatics |
Understanding Racial Disparities, Genetic Risk and Phenotypes in Patients with Hypertrophic Cardiomyopathy using Machine Learning Algorithms
The current research interests include studying the interplay between genetics, race, social determinants of health and outcomes in patients with hypertrophic cardiomyopathy followed at Boston Medical Center, the largest safety net hospital in New England. The group aims to study the use of novel machine learning algorithms to help improve the diagnosis of hypertrophic cardiomyopathy using echocardiography.
Meet the Team
 Dr. Monica Ahluwalia received her MD at Robert Wood Johnson Medical School with Distinction in Research, and completed her Internal Medicine Residency, Cardiovascular Medicine Fellowship and Advanced Heart Failure & Transplant Cardiology Fellowship at University of Pennsylvania, NYU School of Medicine and Brigham and Women’s Hospital, respectively, where she received the Baughman Award of Clinical Excellence. Dr. Ahluwalia is currently an Assistant Professor of Cardiovascular Medicine at the Chobanian and Avedisian School of Medicine and Staff Cardiologist at the Boston Medical Center Boston, MA where she is medical director of the Inherited Cardiovascular Diseases Program. She is also an Advanced Heart Failure & Transplant Cardiologist at Brigham and Women’s Hospital in Boston, MA. Outside the University, she is a member of societies including American College of Cardiology, American Heart Association and Heart Failure Society of America.
Dr. Monica Ahluwalia received her MD at Robert Wood Johnson Medical School with Distinction in Research, and completed her Internal Medicine Residency, Cardiovascular Medicine Fellowship and Advanced Heart Failure & Transplant Cardiology Fellowship at University of Pennsylvania, NYU School of Medicine and Brigham and Women’s Hospital, respectively, where she received the Baughman Award of Clinical Excellence. Dr. Ahluwalia is currently an Assistant Professor of Cardiovascular Medicine at the Chobanian and Avedisian School of Medicine and Staff Cardiologist at the Boston Medical Center Boston, MA where she is medical director of the Inherited Cardiovascular Diseases Program. She is also an Advanced Heart Failure & Transplant Cardiologist at Brigham and Women’s Hospital in Boston, MA. Outside the University, she is a member of societies including American College of Cardiology, American Heart Association and Heart Failure Society of America.
 Dr. Jacques Kpodonu is currently practicing as an NI- funded cardiac surgeon/scientist at Beth Israel Deaconess Medical Center (BIDMC), Assistant Professor of Surgery (Harvard Medical School) and a recent recipient of the 2022 biodiversity fellow leadership program. His research interests are focused at the intersection of cardiac surgery, data science and global health to address cardiovascular health disparities. Along with Emmanuel Agu, a computer science Professor from Worcester Polytechnic Institute, he is co-director of the Cardiovascular Artificial Intelligence Network (CAIN) a network of global researchers with expertise in cardiology, cardiac surgery, global health and data science focused the use of Artificial Intelligence (AI) to democratize cardiovascular care globally particularly in Low- and Middle-Income Countries.
Dr. Jacques Kpodonu is currently practicing as an NI- funded cardiac surgeon/scientist at Beth Israel Deaconess Medical Center (BIDMC), Assistant Professor of Surgery (Harvard Medical School) and a recent recipient of the 2022 biodiversity fellow leadership program. His research interests are focused at the intersection of cardiac surgery, data science and global health to address cardiovascular health disparities. Along with Emmanuel Agu, a computer science Professor from Worcester Polytechnic Institute, he is co-director of the Cardiovascular Artificial Intelligence Network (CAIN) a network of global researchers with expertise in cardiology, cardiac surgery, global health and data science focused the use of Artificial Intelligence (AI) to democratize cardiovascular care globally particularly in Low- and Middle-Income Countries.
 Dr. Emmanuel Agu is currently an NIH-funded professor and scientist in the computer science department at WPI. His research interests are in the areas of computer graphics, mobile computing, and wireless networks. He is especially interested in research into how to use a smartphone as a platform to deliver better healthcare. In collaboration with researchers at WPI and Dr. Jacques Kpodonu, he developed a machine/deep learning tool to estimate left ventricular ejection fraction on echocardiograms. He currently co-directs the Cardiovascular Artificial Intelligence Network (CAIN) with Dr. Kpodonu
Dr. Emmanuel Agu is currently an NIH-funded professor and scientist in the computer science department at WPI. His research interests are in the areas of computer graphics, mobile computing, and wireless networks. He is especially interested in research into how to use a smartphone as a platform to deliver better healthcare. In collaboration with researchers at WPI and Dr. Jacques Kpodonu, he developed a machine/deep learning tool to estimate left ventricular ejection fraction on echocardiograms. He currently co-directs the Cardiovascular Artificial Intelligence Network (CAIN) with Dr. Kpodonu
Funded by CAMed |
Profiling of Vulnerable Neurons During the Prodromal Stage of Alzheimer’s Disease
杜
Meet the Team
 Dr. Jean-Pierre Roussarie is an Assistant Professor in the Anatomy & Neurobiology department at the Boston University Chobanian & Avedisian School of Medicine. After studying engineering in Paris, he did a PhD in neurovirology at the Pasteur Institute. He then moved to New York for his postdoctoral training in the laboratory of Dr. Paul Greengard. He started his lab at BU in 2021. His lab studies selective neuronal vulnerability in Alzheimer’s disease.
Dr. Jean-Pierre Roussarie is an Assistant Professor in the Anatomy & Neurobiology department at the Boston University Chobanian & Avedisian School of Medicine. After studying engineering in Paris, he did a PhD in neurovirology at the Pasteur Institute. He then moved to New York for his postdoctoral training in the laboratory of Dr. Paul Greengard. He started his lab at BU in 2021. His lab studies selective neuronal vulnerability in Alzheimer’s disease.
 Dr. Thor D. Stein completed his undergraduate and graduate studies at the University of Wisconsin- Madison where he earned his MD and PhD (neuroscience) degrees. He completed residency training in pathology and fellowship training in neuropathology at Massachusetts General Hospital. He is currently Associate Professor of Pathology at Boston University Chobanian & Avedisian School of Medicine.
Dr. Thor D. Stein completed his undergraduate and graduate studies at the University of Wisconsin- Madison where he earned his MD and PhD (neuroscience) degrees. He completed residency training in pathology and fellowship training in neuropathology at Massachusetts General Hospital. He is currently Associate Professor of Pathology at Boston University Chobanian & Avedisian School of Medicine.
 Dr. Chao Zhang is an Assistant Professor of Computational Biomedicine in the Department of Medicine at Boston University School of Medicine. As an interdisciplinary researcher, he has a background in systems biology, statistics, machine learning, software engineering, and especially in next-generation sequencing analysis. His current research is centered around the investigation of aging-related and chronic diseases, including chronic kidney disease, Alzheimer’s disease, and degenerative joint diseases. Through the use of computational methods, Dr. Zhang aims to develop innovative deep-learning approaches to analyze the single cell level molecular data, providing valuable insights into disease mechanisms. Furthermore, he integrates clinical data with molecular data to enhance disease diagnosis and treatment strategies.
Dr. Chao Zhang is an Assistant Professor of Computational Biomedicine in the Department of Medicine at Boston University School of Medicine. As an interdisciplinary researcher, he has a background in systems biology, statistics, machine learning, software engineering, and especially in next-generation sequencing analysis. His current research is centered around the investigation of aging-related and chronic diseases, including chronic kidney disease, Alzheimer’s disease, and degenerative joint diseases. Through the use of computational methods, Dr. Zhang aims to develop innovative deep-learning approaches to analyze the single cell level molecular data, providing valuable insights into disease mechanisms. Furthermore, he integrates clinical data with molecular data to enhance disease diagnosis and treatment strategies.
2022 Integrated Pilot Grant Awardees
Funded by BMC |
Pilot Implementation of an Automated and Personalized Risk Prediction Tool of Life Threatening Mass Effect After Middle Cerebral Artery Stroke
Th
Meet the Team
 Dr. Charlene J. Ong is an Assistant Professor of Neurology and Neurosurgery at Boston University, a lecturer at Harvard Medical School, and a clinical neurointensive care physician at Boston Medical Center. She received her undergraduate degree at University of Pennsylvania, her MD at Columbia University and her Master’s in Population Health Sciences at Washington University School of Medicine. Her research focuses on data-driven tools that support clinical decision making and optimize outcome after acute brain injury. She has received foundational support from the NIH in the form of a K23, the American Brain Foundation, Philips-MIT, the Peter Paul Career Development Committee, and the Clinical and 转化科学澳门威尼斯人注册网站研究所 at Boston University. Her aim is to become an independent investigator in data-driven strategies to support clinical decision making and optimize outcomes after acute brain injury.
Dr. Charlene J. Ong is an Assistant Professor of Neurology and Neurosurgery at Boston University, a lecturer at Harvard Medical School, and a clinical neurointensive care physician at Boston Medical Center. She received her undergraduate degree at University of Pennsylvania, her MD at Columbia University and her Master’s in Population Health Sciences at Washington University School of Medicine. Her research focuses on data-driven tools that support clinical decision making and optimize outcome after acute brain injury. She has received foundational support from the NIH in the form of a K23, the American Brain Foundation, Philips-MIT, the Peter Paul Career Development Committee, and the Clinical and 转化科学澳门威尼斯人注册网站研究所 at Boston University. Her aim is to become an independent investigator in data-driven strategies to support clinical decision making and optimize outcomes after acute brain injury.
 Dr. David Greer is Professor and Chair of the Department of Neurology at Boston University School of Medicine and the Richard B. Slifka Chief of Neurology at Boston Medical Center. Dr. Greer is the editor-in-chief of Seminars in Neurology, and the immediate past editor-in-chief for Neurocritical Care on Call. He has authored more than 300 peer-reviewed manuscripts, reviews, chapters, guidelines and books. His research interests include predicting recovery from coma after cardiac arrest, brain death, and multiple stroke-related topics, including acute stroke treatment, temperature modulation and stroke prevention.
Dr. David Greer is Professor and Chair of the Department of Neurology at Boston University School of Medicine and the Richard B. Slifka Chief of Neurology at Boston Medical Center. Dr. Greer is the editor-in-chief of Seminars in Neurology, and the immediate past editor-in-chief for Neurocritical Care on Call. He has authored more than 300 peer-reviewed manuscripts, reviews, chapters, guidelines and books. His research interests include predicting recovery from coma after cardiac arrest, brain death, and multiple stroke-related topics, including acute stroke treatment, temperature modulation and stroke prevention.
SNAP: Supportive Noninvasive Ventilation for Acute Chest Syndrome Prevention for Hospitalized Children with Sickle Cell Disease
博士
Meet the Team
 Dr. Robyn Cohen is the director of the Division of Pediatric Pulmonary and Allergy at Boston Medical Center and Associate Professor of Pediatrics at Boston University School of Medicine. She went to medical school at the Albert Einstein College of Medicine and did her residency in Social Pediatrics at the Children’s Hospital at Montefiore. She completed her pediatric pulmonary fellowship at Boston Children’s Hospital, then went on to pursue a post-doctoral research fellowship in Respiratory Epidemiology at the Channing Laboratory (now the Channing Division of Network Medicine) at Brigham and Women’s Hospital, which included an MPH in Clinical Effectiveness at the Harvard School of Public Health. Her clinical and research interests are in respiratory health disparities in children with a focus on pediatric asthma and sickle cell lung disease.
Dr. Robyn Cohen is the director of the Division of Pediatric Pulmonary and Allergy at Boston Medical Center and Associate Professor of Pediatrics at Boston University School of Medicine. She went to medical school at the Albert Einstein College of Medicine and did her residency in Social Pediatrics at the Children’s Hospital at Montefiore. She completed her pediatric pulmonary fellowship at Boston Children’s Hospital, then went on to pursue a post-doctoral research fellowship in Respiratory Epidemiology at the Channing Laboratory (now the Channing Division of Network Medicine) at Brigham and Women’s Hospital, which included an MPH in Clinical Effectiveness at the Harvard School of Public Health. Her clinical and research interests are in respiratory health disparities in children with a focus on pediatric asthma and sickle cell lung disease.
 Dr. Caitlin Neri is an academic pediatric hematologist at Boston Medical Center (BMC) and Boston University School of Medicine (BUSM) with particular interest in treating children with sickle cell disease, multimodal pain management strategies, and supportive care of children with sickle cell disease. She clinically cares for patients in BMC’s Pediatric Sickle Cell program and directs the multidisciplinary Pediatric Pain Clinic at BMC. She is also an Assistant Professor of Pediatrics at BUSM, where she directs a second-year medical student course in hematology and is currently overseeing the hematology content for the upcoming BUSM curriculum redesign.
Dr. Caitlin Neri is an academic pediatric hematologist at Boston Medical Center (BMC) and Boston University School of Medicine (BUSM) with particular interest in treating children with sickle cell disease, multimodal pain management strategies, and supportive care of children with sickle cell disease. She clinically cares for patients in BMC’s Pediatric Sickle Cell program and directs the multidisciplinary Pediatric Pain Clinic at BMC. She is also an Assistant Professor of Pediatrics at BUSM, where she directs a second-year medical student course in hematology and is currently overseeing the hematology content for the upcoming BUSM curriculum redesign.
Toward Noninvasive Imaging Biomarkers of Drug-resistant Epilepsy
The aim is to investigate a novel noninvasive imaging method for the evaluation of blood-brain barrier permeability. Such findings would improve our knowledge about mechanisms of treatment resistance and the care of people with epilepsy. (Award co-funded with the BU CTSI)
Meet the Team
 Dr. Myriam Abdennadher is an Assistant Professor of Medicine in the department of neurology at Boston University School of Medicine (BUSM). Sheis a board-certified neurologist, clinical neurophysiologist, and epileptologist, with a focus on drug-resistant epilepsy, neuroimaging of epilepsy, seizure focus localization and epilepsy surgery. Her research focuses on neurophysiologic and brain imaging methods in drug-resistant epilepsy. She is particularly interested in non-invasive MRI methods to better understand drug resistance and to define seizure zone. Her goal is to improve seizure control and quality of life in patients whose seizures don’t respond to medications and who may benefit from surgical treatment.
Dr. Myriam Abdennadher is an Assistant Professor of Medicine in the department of neurology at Boston University School of Medicine (BUSM). Sheis a board-certified neurologist, clinical neurophysiologist, and epileptologist, with a focus on drug-resistant epilepsy, neuroimaging of epilepsy, seizure focus localization and epilepsy surgery. Her research focuses on neurophysiologic and brain imaging methods in drug-resistant epilepsy. She is particularly interested in non-invasive MRI methods to better understand drug resistance and to define seizure zone. Her goal is to improve seizure control and quality of life in patients whose seizures don’t respond to medications and who may benefit from surgical treatment.
 Dr. Ning Hua is an Assistant Professor of Radiology at Boston University School of Medicine (BUSM). She is an MRI physicist and expert in neuroimaging and cardiovascular imaging. Her current research focuses on using advanced perfusion MRI methods to study vascular dysfunction in brain pathologies. She is particularly interested in the microvascular contribution to epilepsy, Alzheimer’s disease, related neurodegenerations, and the aging brain. Her goal is to develop novel imaging markers for evaluating blood-brain barrier integrity and microcirculation status (permeability, perfusion, flow rate, curvature, etc.) in both clinical and preclinical settings, and ultimately to advance relevant diagnosis and treatments.
Dr. Ning Hua is an Assistant Professor of Radiology at Boston University School of Medicine (BUSM). She is an MRI physicist and expert in neuroimaging and cardiovascular imaging. Her current research focuses on using advanced perfusion MRI methods to study vascular dysfunction in brain pathologies. She is particularly interested in the microvascular contribution to epilepsy, Alzheimer’s disease, related neurodegenerations, and the aging brain. Her goal is to develop novel imaging markers for evaluating blood-brain barrier integrity and microcirculation status (permeability, perfusion, flow rate, curvature, etc.) in both clinical and preclinical settings, and ultimately to advance relevant diagnosis and treatments.
Funded by GSDM |
Evaluation of Genotype and Phenotype in a New Hypomaturation Type of Human Rare Dental Disease, Amelogenesis Imperfecta
来
Funded by DOM |
Characterizing Care Coordination in Pulmonary Hypertension: A Qualitative Study
聚氨酯
Meet the Team
 Dr. Kari R. Gillmeyer is an Assistant Professor of Medicine at Boston University School of Medicine. She is a pulmonary and critical care physician and health services researcher whose work focuses on improving care delivery, quality of care, and outcomes for patients living with PH. Her NIH and foundation funded research utilizes quantitative, qualitative, and mixed method approaches to investigate a wide range of topics within the field of PH, such as drivers and outcomes of guideline-discordant PH care, limitations of administrative data sources in PH research, and prevalence and effects of multisystem PH care. This body of work has identified significant gaps in our understanding of pulmonary hypertension care delivery, including how care is organized and coordinated across the U.S. Her prior research serves as the motivation for this CTSI Pilot Award.
Dr. Kari R. Gillmeyer is an Assistant Professor of Medicine at Boston University School of Medicine. She is a pulmonary and critical care physician and health services researcher whose work focuses on improving care delivery, quality of care, and outcomes for patients living with PH. Her NIH and foundation funded research utilizes quantitative, qualitative, and mixed method approaches to investigate a wide range of topics within the field of PH, such as drivers and outcomes of guideline-discordant PH care, limitations of administrative data sources in PH research, and prevalence and effects of multisystem PH care. This body of work has identified significant gaps in our understanding of pulmonary hypertension care delivery, including how care is organized and coordinated across the U.S. Her prior research serves as the motivation for this CTSI Pilot Award.
 Dr. Renda Soylemez Wiener is a Professor of Medicine at Boston University School of Medicine. She is a pulmonary and critical care physician, health services researcher, and implementation scientist. The goal of her research is to improve communication and decision- making between patients and clinicians, de-implement low value, potentially harmful practices, and implement patient-centered programs. She applies mixed methods to approach complex research questions from multiple angles and has experience conducting quantitative analyses of administrative databases, qualitative analysis, survey research, and evaluations of implementation. Her work has been highly influential; she has published 5 articles that are in the top 1% of most highly cited papers in the field of clinical medicine (per Web of Science) and has an overall h-index of 38, with over 8000 citations to her work (per Google Scholar). She brings extensive, relevant content and methodological expertise to this multidisciplinary CTSI Pilot Award.
Dr. Renda Soylemez Wiener is a Professor of Medicine at Boston University School of Medicine. She is a pulmonary and critical care physician, health services researcher, and implementation scientist. The goal of her research is to improve communication and decision- making between patients and clinicians, de-implement low value, potentially harmful practices, and implement patient-centered programs. She applies mixed methods to approach complex research questions from multiple angles and has experience conducting quantitative analyses of administrative databases, qualitative analysis, survey research, and evaluations of implementation. Her work has been highly influential; she has published 5 articles that are in the top 1% of most highly cited papers in the field of clinical medicine (per Web of Science) and has an overall h-index of 38, with over 8000 citations to her work (per Google Scholar). She brings extensive, relevant content and methodological expertise to this multidisciplinary CTSI Pilot Award.
 Dr. A. Rani Elwy is an Associate Professor at the Warren Alpert Medical School of Brown University in the Department of Psychiatry and Human Behavior, and Adjunct Associate Professor of Boston University School of Public Health in the Department of Health Law,Policy, and Management. She is a renowned expert in mixed methods research, stakeholder engagement methods, and implementation science. Her work focuses on building stakeholder and leadership buy-in for national policy implementation across large integrated healthcare systems; evaluating and disseminating evidence-based complementary and integrative health therapies to treat PTSD, depression, and chronic pain; social network analysis; and implementation outcome assessment. With this expertise, she will help ensure that findings from this pilot work can directly inform the development of an intervention in the next stage of research.
Dr. A. Rani Elwy is an Associate Professor at the Warren Alpert Medical School of Brown University in the Department of Psychiatry and Human Behavior, and Adjunct Associate Professor of Boston University School of Public Health in the Department of Health Law,Policy, and Management. She is a renowned expert in mixed methods research, stakeholder engagement methods, and implementation science. Her work focuses on building stakeholder and leadership buy-in for national policy implementation across large integrated healthcare systems; evaluating and disseminating evidence-based complementary and integrative health therapies to treat PTSD, depression, and chronic pain; social network analysis; and implementation outcome assessment. With this expertise, she will help ensure that findings from this pilot work can directly inform the development of an intervention in the next stage of research.
This CTSI pilot grant, building on recent preliminary findings of activated receptors on hematopoietic stem cells affected by the JAK2V617F mutation, focuses on novel therapeutic approaches to reducing the burden of the malignant clone in primary myelofibrosis. This project will be the crucial first step in the pursuit of translational application of this research program.
Meet the Team
博士
Impact of IgA and IgM upon Nasal Microbiota in Common Variable Immunodeficiency
Common variable immunodeficiency (CVID) is the most common symptomatic primary immunodeficiency. Respiratory ailments are the most frequent complications of CVID, with chronic pulmonary disease developing in 30-60% and even more experiencing frequent acute respiratory infections. This project aims to establish cutting-edge approaches to study pulmonary biology in CVID and apply novel bioinformatics strategies in these patients to study complex interactions among microbes and host cells directly in the respiratory tract. This will include evaluating several ways to sample gene expression in the respiratory tract and apply cutting-edge computational approaches to analyzing the complex data derived from these approaches.
Meet the Team
 Dr. Paul J. Maglione is a physician-scientist in the Pulmonary Center of Boston University School of Medicine. Dr. Maglione studies human B cell biology, particularly in how it relates to and is informed by primary immunodeficiency disorders. His research program utilizes high throughput strategies such as RNA sequencing, B cell receptor repertoire analysis, and seromics together with cell culture, immunofluorescence, and information from the patient medical record to conduct bedside-to-bench studies on immunodeficiency disorders. In addition to running a research laboratory, Dr. Maglione provides clinical care for patients with immunodeficiency disorders and has particular interest and expertise in common variable immunodeficiency (CVID) and its related complications, including chronic lung disease.
Dr. Paul J. Maglione is a physician-scientist in the Pulmonary Center of Boston University School of Medicine. Dr. Maglione studies human B cell biology, particularly in how it relates to and is informed by primary immunodeficiency disorders. His research program utilizes high throughput strategies such as RNA sequencing, B cell receptor repertoire analysis, and seromics together with cell culture, immunofluorescence, and information from the patient medical record to conduct bedside-to-bench studies on immunodeficiency disorders. In addition to running a research laboratory, Dr. Maglione provides clinical care for patients with immunodeficiency disorders and has particular interest and expertise in common variable immunodeficiency (CVID) and its related complications, including chronic lung disease.
Novel Urinary Acute Kidney Injury (AKI) Diagnostic for Cardiothoracic Surgery Patients
In collaboration with Dr. Niloo M. Edwards, they will assess the sensitivity and specificity of urinary nucleophosmin (NPM) and phosphorylated-NPM (p-NPM) as non-invasive markers of renal damage in BMC patients undergoing cardiac surgery at high risk for acute kidney injury (AKI)
 Dr. Steven C. Borkan is an Associate Professor of Medicine and Co-Director of the MD-PhD Training Program at Boston University School of Medicine, and an attending physician in the Renal Section at Boston Medical Center. His interests include education of medical students and house staff, basic research on the cellular mechanisms of acute kidney injury (AKI), and the care of underserved patients with renal disease. Dr. Borkan is the senior author of numerous publications in the pathobiology of renal cell injury during AKI and a NIH-funded Principal Investigator. He hopes to harness insights into the pathogenesis of regulated cell death to detect and ultimately treat ischemic acute kidney injury in cardiac surgery patients at high risk for this devastating complication.
Dr. Steven C. Borkan is an Associate Professor of Medicine and Co-Director of the MD-PhD Training Program at Boston University School of Medicine, and an attending physician in the Renal Section at Boston Medical Center. His interests include education of medical students and house staff, basic research on the cellular mechanisms of acute kidney injury (AKI), and the care of underserved patients with renal disease. Dr. Borkan is the senior author of numerous publications in the pathobiology of renal cell injury during AKI and a NIH-funded Principal Investigator. He hopes to harness insights into the pathogenesis of regulated cell death to detect and ultimately treat ischemic acute kidney injury in cardiac surgery patients at high risk for this devastating complication.
Funded by CTSI |
Allostatic Load and Physical Activity as Modulators of Racial Disparities in Neurocognitive Aging in the Framingham Heart Study Cohort
Utilizing the Framingham Heart Study Brain Aging Program dataset, the goal of the CTSI pilot grant award is to examine whether racial disparities in allostatic load, the physiological ‘wear and tear’ of the body in response to chronic stress, can account for racial disparities in neurocognitive aging between Black and White older adults and whether physical activity can attenuate these neurocognitive disparities by lowering allostatic load.
 Dr. Karin Schon is a cognitive neuroscientist and Assistant Professor at the Department of Anatomy & Neurobiology. She received her Ph.D. in Psychology from Boston University in 2005 and her undergraduate degree in psychology from the University of Hamburg in Germany. She is a past recipient of a K99/R00 Pathway to Independence Award from the National Institute on Aging. Dr. Schon’s research focuses on modulators of the medial temporal hippocampal memory system, including exercise, psychosocial and physiological stress, and aging. Her most recent research examines the impact of racism burden on brain and mental health across the lifespan in Black Americans.
Dr. Karin Schon is a cognitive neuroscientist and Assistant Professor at the Department of Anatomy & Neurobiology. She received her Ph.D. in Psychology from Boston University in 2005 and her undergraduate degree in psychology from the University of Hamburg in Germany. She is a past recipient of a K99/R00 Pathway to Independence Award from the National Institute on Aging. Dr. Schon’s research focuses on modulators of the medial temporal hippocampal memory system, including exercise, psychosocial and physiological stress, and aging. Her most recent research examines the impact of racism burden on brain and mental health across the lifespan in Black Americans.
A Prospective Mixed Methods Study of Maternal and Child Well-being and Risk of Relapse in the First Year Postpartum
Th
Meet the Team
 Dr. Mei A. Elansary is an Assistant Professor of Pediatrics at Boston University School of Medicine. Dr. Elansary has special interests in the evaluation and treatment of children with concerns about behavior and development in the context of maternal adversity. She works as the Developmental Behavioral Pediatrician for the SOFAR (Supporting Our Families through Addiction and Recovery) Program. As an early career researcher, Dr. Elansary is interested
Dr. Mei A. Elansary is an Assistant Professor of Pediatrics at Boston University School of Medicine. Dr. Elansary has special interests in the evaluation and treatment of children with concerns about behavior and development in the context of maternal adversity. She works as the Developmental Behavioral Pediatrician for the SOFAR (Supporting Our Families through Addiction and Recovery) Program. As an early career researcher, Dr. Elansary is interested
in interventions that mitigate the intergenerational effects of maternal post–traumatic stress on child development. Dr. Elansary received her medical degree from Yale University School of Medicine. She completed pediatrics training at the Boston Combined Residency Program and completed subspeciality training in Developmental and Behavioral Pediatrics at Boston Children’s Hospital prior to joining the faculty at Boston University.
Biomagnetic Measurements of Animal Hearts with Novel Magnetic Sensors
Professor Bishop and Dr. Josh Javor, a postdoc in his lab, recently invented a small, low-cost, low power magnetic sensor with very high sensitivity in the range of low frequency biomagnetic fields. Together they were awarded the Pilot Grant from Boston University’s Clinical and 转化科学澳门威尼斯人注册网站研究所 (CTSI) to detect the magnetic fields from animal hearts. If successful, their work may lead to the development of a non-contact, radiation-free cardiac diagnostic imaging tool that can increase the confidence of cardiologists during diagnosis and in the safe discharge of patients experiencing chest pain.
 Dr. David Bishop is currently the Director of CELL-MET, an NSF ERC, and Head of the Division of Materials Science and Engineering at Boston University. He is also a Professor of Physics, Professor of Mechanical Engineering, Professor of Electrical and Computer Engineering, Professor of Biomedical Engineering and Professor of Materials Science and Engineering at BU. Prior to joining BU, he was the Chief Technology Officer (CTO) and Chief Operating Officer (COO) of Lucent Technologies, Bell Labs. Dr. Bishop graduated from Syracuse University with a B.S. in Physics. He received an M.S. and Ph.D. in Physics from Cornell University. Professor Bishop has over 23,000 citations on published scientific articles, 50 issued patents, and is a member of the National Academy of Inventors and the National Academy of Engineering.
Dr. David Bishop is currently the Director of CELL-MET, an NSF ERC, and Head of the Division of Materials Science and Engineering at Boston University. He is also a Professor of Physics, Professor of Mechanical Engineering, Professor of Electrical and Computer Engineering, Professor of Biomedical Engineering and Professor of Materials Science and Engineering at BU. Prior to joining BU, he was the Chief Technology Officer (CTO) and Chief Operating Officer (COO) of Lucent Technologies, Bell Labs. Dr. Bishop graduated from Syracuse University with a B.S. in Physics. He received an M.S. and Ph.D. in Physics from Cornell University. Professor Bishop has over 23,000 citations on published scientific articles, 50 issued patents, and is a member of the National Academy of Inventors and the National Academy of Engineering.
Development and In Vivo Efficacy of Small-Molecule IL-4 Inhibitors
在
Meet the Team
 Dr. Arturo J. Vegas is a Peter Paul Career Development Professor at Boston University. He is appointed in the Department of Chemistry and has affiliations with the Department of Biomedical Engineering and the Materials Science and Engineering Division. He is a core faculty member of the BU Center for Molecular Discovery, the BU Nanotechnology Innovation Center, the Biological Design Center, and is Co-Director of the Translational Research in Biomaterials Training Program. He has also received a New Innovator Type 1 Diabetes Pathfinder Award from the NIH. Arturo received his BA in Biology from Cornell University and a PhD in Chemistry from Harvard University. Research in the Vegas lab focuses on developing novel tissue-targeted drug delivery systems and new therapies for immunomodulation for cancer, type 1 diabetes, and immune-mediated disorders.
Dr. Arturo J. Vegas is a Peter Paul Career Development Professor at Boston University. He is appointed in the Department of Chemistry and has affiliations with the Department of Biomedical Engineering and the Materials Science and Engineering Division. He is a core faculty member of the BU Center for Molecular Discovery, the BU Nanotechnology Innovation Center, the Biological Design Center, and is Co-Director of the Translational Research in Biomaterials Training Program. He has also received a New Innovator Type 1 Diabetes Pathfinder Award from the NIH. Arturo received his BA in Biology from Cornell University and a PhD in Chemistry from Harvard University. Research in the Vegas lab focuses on developing novel tissue-targeted drug delivery systems and new therapies for immunomodulation for cancer, type 1 diabetes, and immune-mediated disorders.
 Dr. Felicia Chen is an Assistant Professor of Medicine at Boston University School of Medicine and a pulmonary and critical care physician at Boston Medical Center. Her research interest is to uncover the molecular and cellular mechanisms that regulate lung development, homeostasis, and injury repair. Recently, her projects focused on the impact of vitamin A signaling in airway physiology, smooth muscle phenotype, and lung microbiota interactions. In addition to her clinical and research activities, she is active in the education of predoctoral students, medical residents, and pulmonary and critical care fellows at Boston University and Boston Medical Center. Dr. Chen earned her medical degree from Albany Medical College and completed her residency and fellowship training at Boston University School of Medicine.
Dr. Felicia Chen is an Assistant Professor of Medicine at Boston University School of Medicine and a pulmonary and critical care physician at Boston Medical Center. Her research interest is to uncover the molecular and cellular mechanisms that regulate lung development, homeostasis, and injury repair. Recently, her projects focused on the impact of vitamin A signaling in airway physiology, smooth muscle phenotype, and lung microbiota interactions. In addition to her clinical and research activities, she is active in the education of predoctoral students, medical residents, and pulmonary and critical care fellows at Boston University and Boston Medical Center. Dr. Chen earned her medical degree from Albany Medical College and completed her residency and fellowship training at Boston University School of Medicine.
Developing Tools for Early Detection and Prevention of Lead Take Home
勒
Meet the Team
 Dr. Diana M. Cellabos is an Assistant Professor at Boston University School of Public Health. Her life’s passion is to address health disparities by identifying environmental factors that cause disease, injury, or impairment. These factors range from emerging hazards related to new technologies, to known hazards that are transferred to vulnerable populations including workers in small businesses, minorities, and workers in developing economies. Her research aims to better understand health effects from exposure to complex mixtures to uncovering and addressing the disproportionate burden of exposure in vulnerable populations. She is motivated by interdisciplinary and collaborative research projects to understand and prevent health effects of environmental and occupational contaminants in the United States and abroad. She has expertise in the development, coordination and analysis of highly complex environmental and biological sampling techniques, including the development of new sampling methodologies.
Dr. Diana M. Cellabos is an Assistant Professor at Boston University School of Public Health. Her life’s passion is to address health disparities by identifying environmental factors that cause disease, injury, or impairment. These factors range from emerging hazards related to new technologies, to known hazards that are transferred to vulnerable populations including workers in small businesses, minorities, and workers in developing economies. Her research aims to better understand health effects from exposure to complex mixtures to uncovering and addressing the disproportionate burden of exposure in vulnerable populations. She is motivated by interdisciplinary and collaborative research projects to understand and prevent health effects of environmental and occupational contaminants in the United States and abroad. She has expertise in the development, coordination and analysis of highly complex environmental and biological sampling techniques, including the development of new sampling methodologies.
 Dr. Jennifer Greif Green is an associate professor in special education and a child clinical psychologist. Her research focuses on supporting students with emotional/behavioral disorders and bullying prevention. Within these lines of research, she studies teacher identification of students with mental health needs, racial/ethnic disparities in mental health service access, and youth bullying involvement.
Dr. Jennifer Greif Green is an associate professor in special education and a child clinical psychologist. Her research focuses on supporting students with emotional/behavioral disorders and bullying prevention. Within these lines of research, she studies teacher identification of students with mental health needs, racial/ethnic disparities in mental health service access, and youth bullying involvement.
 Dr. Noah Buncher is a pediatrician in Primary Care Pediatrics at Boston Medical Center and Assistant Professor of pediatrics at Boston University School of Medicine. His interests are varied and include adverse childhood experiences, refugee medicine, global health, and motivational interviewing. During his residency, he was awarded an AAP CATCH Grant to help establish one of Connecticut’s only dedicated pediatric global health clinics designed to provide culturally sensitive, comprehensive care to newly arrived refugee children, help transition these children to a medical home, and connect refugee families to community resources. Dr. Buncher was a Visiting Resident Scholar with Baylor International Pediatric Aids Initiative, providing clinical care to HIV positive children, adolescents, and their families at Baylor College of Medicine Children’s Foundation – Lesotho.
Dr. Noah Buncher is a pediatrician in Primary Care Pediatrics at Boston Medical Center and Assistant Professor of pediatrics at Boston University School of Medicine. His interests are varied and include adverse childhood experiences, refugee medicine, global health, and motivational interviewing. During his residency, he was awarded an AAP CATCH Grant to help establish one of Connecticut’s only dedicated pediatric global health clinics designed to provide culturally sensitive, comprehensive care to newly arrived refugee children, help transition these children to a medical home, and connect refugee families to community resources. Dr. Buncher was a Visiting Resident Scholar with Baylor International Pediatric Aids Initiative, providing clinical care to HIV positive children, adolescents, and their families at Baylor College of Medicine Children’s Foundation – Lesotho.
Disparities in Time to Diagnosis of Ankylosing Spondylitis
The team will use analytic tools developed by Observational Health Data Sciences and Informatics (ODHSI) to study the time to diagnosis among people with ankylosing spondylitis, a condition that is often associated with 6-8 years from the onset of back/joint pain to diagnosis. They will study whether the time to diagnosis varies according to gender, race, or ethnicity. By using Boston Medical Center data in the Observational Medical Outcomes Partnership (OMOP) structure, the team will be able to expand future analyses to other datasets that use the same model.
Meet the Team
 Dr. Maureen Dubreuil is a rheumatologist who specializes in spondyloarthritis. Her work focuses on comorbidities and pharmacoepidemiology of spondyloarthritis.
Dr. Maureen Dubreuil is a rheumatologist who specializes in spondyloarthritis. Her work focuses on comorbidities and pharmacoepidemiology of spondyloarthritis.
Dr. S. Reza Jafarzadeh is an epidemiologist with a research interest on the application of causal inference methods in observational studies of rheumatic diseases using OHDSI tools and data standards.
Gut health, Micronutrient Status, and Linear Growth Among Infants in Lusaka, Zambia
联合国
Meet the Team
 Dr. Lindsey M. Locks is an Assistant Professor in the Departments of Health Sciences (Sargent College) and Global Health (School of Public Health). She directs the Sargent College Global Nutrition Lab, and her research focuses on undernutrition in women and children in low- and middle-income countries. Her current research focuses on the role of human milk, micronutrients, infant and young child feeding practices in infant gut health and pediatric growth. She has 15 years of experience in global nutrition research and programs in sub-Saharan Africa and South Asia. She holds a ScD in Nutritional Epidemiology from the Harvard Chan School of Public Health and a MPH in Epidemiology and Global Health from the Columbia University Mailman School of Public Health.
Dr. Lindsey M. Locks is an Assistant Professor in the Departments of Health Sciences (Sargent College) and Global Health (School of Public Health). She directs the Sargent College Global Nutrition Lab, and her research focuses on undernutrition in women and children in low- and middle-income countries. Her current research focuses on the role of human milk, micronutrients, infant and young child feeding practices in infant gut health and pediatric growth. She has 15 years of experience in global nutrition research and programs in sub-Saharan Africa and South Asia. She holds a ScD in Nutritional Epidemiology from the Harvard Chan School of Public Health and a MPH in Epidemiology and Global Health from the Columbia University Mailman School of Public Health.
 Dr. Peter Rockers is an Associate Professor in the Department of Global Health at the Boston University School of Public Health. He is the Director of the Global Health Program Design, Monitoring & Evaluation certificate within the MPH program. Dr. Rockers’ research is primarily concerned with understanding and improving child development outcomes in low- and middle- income countries. He uses epidemiologic methods to investigate biological and social determinants of child development. He also uses experimental methods to test the impacts of interventions that aim to improve child development. Dr. Rockers is particularly interested in the relationship between poverty and neurodevelopment
Dr. Peter Rockers is an Associate Professor in the Department of Global Health at the Boston University School of Public Health. He is the Director of the Global Health Program Design, Monitoring & Evaluation certificate within the MPH program. Dr. Rockers’ research is primarily concerned with understanding and improving child development outcomes in low- and middle- income countries. He uses epidemiologic methods to investigate biological and social determinants of child development. He also uses experimental methods to test the impacts of interventions that aim to improve child development. Dr. Rockers is particularly interested in the relationship between poverty and neurodevelopment
 Dr. Jacqueline M. Lauer is a Clinical Assistant Professor in the Department of Health Sciences at Boston University. Her research focuses on the environmental contributors to poor growth and development among infants and young children in low-resource settings. She completed a postdoctoral research fellowship at Boston Children’s Hospital where she studied the causes, consequences, and assessment of environmental enteric dysfunction (EED), a subclinical, inflammatory disorder of the small intestine. She completed her PhD in Food Policy and Applied Nutrition from Tufts University’s Friedman School of Nutrition Science and Policy while working as a researcher for USAID’s Feed the Future Innovation Lab for Nutrition in Uganda. She also holds a MPH in International Health and Development and a BSPH from Tulane University.
Dr. Jacqueline M. Lauer is a Clinical Assistant Professor in the Department of Health Sciences at Boston University. Her research focuses on the environmental contributors to poor growth and development among infants and young children in low-resource settings. She completed a postdoctoral research fellowship at Boston Children’s Hospital where she studied the causes, consequences, and assessment of environmental enteric dysfunction (EED), a subclinical, inflammatory disorder of the small intestine. She completed her PhD in Food Policy and Applied Nutrition from Tufts University’s Friedman School of Nutrition Science and Policy while working as a researcher for USAID’s Feed the Future Innovation Lab for Nutrition in Uganda. She also holds a MPH in International Health and Development and a BSPH from Tulane University.
Impact of APOL1 Renal Risk Variants on Placental Function and Racial Disparities in Preeclampsia Risk
有限公司
Meet the Team
 Dr. Wendy Kuohung is the Division Director of Reproductive Endocrinology and Infertility at Boston Medical Center and an Associate Professor of Obstetrics and Gynecology at Boston University School of Medicine. Dr. Kuohung received her medical degree from the Yale University School of Medicine. She completed her Obstetrics and Gynecology residency training at Boston Medical Center and fellowship training in Reproductive Endocrinology and Infertility at Brigham and Women’s Hospital. Dr. Kuohung’s research interests are focused on placental development, infections of the reproductive tract, and reproductive health care disparities. She also has clinical expertise in advanced minimally invasive and robotic gynecologic surgery, female infertility, and fertility preservation. Dr. Kuohung was a former Reproductive Scientist Development Program Scholar and has served as PI on a number of NIH and pharmaceutical research grants since 2003.
Dr. Wendy Kuohung is the Division Director of Reproductive Endocrinology and Infertility at Boston Medical Center and an Associate Professor of Obstetrics and Gynecology at Boston University School of Medicine. Dr. Kuohung received her medical degree from the Yale University School of Medicine. She completed her Obstetrics and Gynecology residency training at Boston Medical Center and fellowship training in Reproductive Endocrinology and Infertility at Brigham and Women’s Hospital. Dr. Kuohung’s research interests are focused on placental development, infections of the reproductive tract, and reproductive health care disparities. She also has clinical expertise in advanced minimally invasive and robotic gynecologic surgery, female infertility, and fertility preservation. Dr. Kuohung was a former Reproductive Scientist Development Program Scholar and has served as PI on a number of NIH and pharmaceutical research grants since 2003.
 Dr. Nader Rahimi is a molecular biologist and currently an Associate Professor at the Department of Pathology and Laboratory Medicine at Boston University. Nader Rahimi has extensively published in the field of signal transduction by receptor tyrosine kinases in particular VEGF receptor tyrosine kinases. His notable works include the demonstration of the differential function of VEGFR-1 and VEGFR-2 in angiogenesis, identification of lysine methylation as a novel mechanism of activation of VEGFR-2, and establishing protein ubiquitination as a major pathway modulating the angiogenic signaling of VEGFR-2. He is also responsible for the discovery of multiple cell surface receptors including, IGPR-1 (TMIGD2), TMIGD1, MINAR1, and MINAR2. His recent work on COVID-19 resulted in the discovery of CD209L and CD209 as novel receptors and vimentin as an attachment factor for SARS-CoV-2.
Dr. Nader Rahimi is a molecular biologist and currently an Associate Professor at the Department of Pathology and Laboratory Medicine at Boston University. Nader Rahimi has extensively published in the field of signal transduction by receptor tyrosine kinases in particular VEGF receptor tyrosine kinases. His notable works include the demonstration of the differential function of VEGFR-1 and VEGFR-2 in angiogenesis, identification of lysine methylation as a novel mechanism of activation of VEGFR-2, and establishing protein ubiquitination as a major pathway modulating the angiogenic signaling of VEGFR-2. He is also responsible for the discovery of multiple cell surface receptors including, IGPR-1 (TMIGD2), TMIGD1, MINAR1, and MINAR2. His recent work on COVID-19 resulted in the discovery of CD209L and CD209 as novel receptors and vimentin as an attachment factor for SARS-CoV-2.
Improve Language Diversity in Clinical Trials
Even though many patients with limited English proficiency (LEP) can be helped through clinical trials, they are often excluded due to a lack of diverse research teams that are able to effectively engage with them. This gap in trial representation translates to a gap in treatment. Boston University School of Social Work (BUSSW) Dr. Martinez will help close this gap by testing and assessing promising engagement strategies with a grant from the Boston University Clinical and 转化科学澳门威尼斯人注册网站研究所 (CTSI). The CTSI Integrated Pilot Grant Program has awarded funds to study CTSI sites including Boston Medical Center, where 31% of patients report a first language other than English.
Advancing language justice in clinical trials requires an investment in systems-level change to support the intentional inclusion of LEP patients. “The enrollment and retention of LEP patients in research requires diverse research teams who are able to communicate, engage, and build trust with limited-English speaking populations,” Sprague Martinez explains. “Solutions that lead to fully representing LEP patients in research may provide increased access to specialized novel treatments only available through trials, and thus serve to bridge racial and ethnic disparities in health.” These steps will create a collection of best practices for medical institutions to adopt.
Meet the Team
Linda Sprague Martinez is associate professor and chair of the Macro Department at the Boston University School of Social Work. She is interested in examining asset-based strategies to tackle health inequities as such community-engaged research (CEnR) approaches like community-based participatory research (CBPR) and youth-led participatory action research (YPAR) are central to her work. Having formerly worked in municipal and state governance, and as an adolescent mental health provider, she brings practical expertise in community collaborations designed to engage diverse communities of color and low-income residents in community planning and intervention development. In 2017 she was a Boston Housing Authority, Center for Community Engagement and Civil Rights, Resident Empowerment Coalition, Resident Empowerment Honoree.
 James Feldman, MD
James Feldman, MD
A member of the research faculty in the Department of Emergency Medicine.Chair of the Boston Medical Center/Boston University Medical Campus IRB Panel Blue and Professor of Emergency Medicine at the Boston University School of Medicine and Director Regulatory Quality and Efficiency BU-CTSI. Research interests have included prehospital emergency medical care, acute cardiac ischemia, cocaine induced cardiac ischemia, public health and human subjects research ethics. Served as President of the Massachusetts College of Emergency Physicians (MACEP) 2008-9. The recipient of several teaching and academic awards including the Pinnacle Award from MACEP (2012), the Mark E. Weinstein MD award for contributions to regional emergency medical services (2012) and the Grant V. Rodkey MD award from the Massachusetts Medical Society (MMS), 2014.
Tr
Rebecca Lobb is the Assistant Director of Community Engagement (CE) for Boston University’s Clinical and Translational Research Institute. She has extensive experience in community-engaged research collaborations with community-based organizations, public health departments, health care organizations, and resident groups to develop sustainable cancer control programs for vulnerable populations
 Chris Sheldrick, PhD
Chris Sheldrick, PhD
Dr. Chris Sheldrick’s research focuses on the science and practice of screening and clinical decision making, spanning from instrument development to implementation and evaluation of screening protocols. Collaborating with Dr. Ellen Perrin, Dr. Sheldrick helped to create the Survey of Wellbeing of Young Children (www.theSWYC.org), a freely available comprehensive screening instrument for young children. Together, they are now completing a study comparing the accuracy of several developmental and behavioral screening instruments that are prominently used in pediatrics. In addition, Dr. Sheldrick has received training in systems science and decision analysis through a KM1 fellowship. Dr. Sheldrick’s current research applies these methods with the goal of helping clinicians improve identify and help children with developmental and behavioral problems in a range of community settings.
Ryan Schroeder
Director, Clinical Research Network at Boston Medical Center (BMC
Over 20 years serving in functional and technical leadership roles in sponsored research administration and clinical research operations at universities and research hospitals. Ryan has led industry sponsored research at UCSF, Cedars-Sinai Medical Center and a California-based community health system, specializing in building high performing, community engaged research programs. Ryan serves as the director of BMC’s Clinical Research Network; an innovative unit started in 2021 that merges community engaged values, removing infrastructural barriers to inclusive research participation, and highly experienced research staffing to manage BMC’s most important health equity-based clinical trials.
Senior Director at Boston Medical Center (BMC), Department of Research Administration.
Jennifer Pamphile is the Community Engagement Specialist for the Community Engagement Program of Boston University’s Clinical and Translational Research Institute. Jennifer has several years of experience in supporting various stakeholders through capacity building and enhancing participatory research knowledge. Previously she worked with the Women’s Health Unit as a bilingual research assistant and community engagement coordinator. Her past work has culminated in the co-creation of public-facing training programs that introduce the audience to Community Engaged Research while improving advocacy, storytelling, and empathic communication skills. She returns to our team from a publishing company where she supported large scale academic- industry collaborations centered around data science. Jennifer received her Bachelor’s of Science in Behavioral Neuroscience and her Master’s of Public Health in Urban Health from Northeastern University.
Riana Howard, MSW (Doctoral RA)
Riana Howard is a first-year doctoral student with an interest in researching how the incorporation of trauma-informed practices into education systems can improve the mental health of marginalized youth and decrease the trauma caused through the education process. She is also interested in researching school discipline practices amongst students of color, carceral logic, and their connection to the school-to-prison pipeline. She earned her MSW degree from California State University, Monterey Bay in 2015. Since graduating, she has been employed in higher education working with non-traditional students (transfer students, student parents, former foster youth students, and formerly incarcerated students), supporting student equity programs, working with Hispanic Serving Institute (HSI) grants, and the African American Resource and Cultural Center. Her work is rooted in educational equity practices and assisting in breaking down systematic barriers in the educational system.
Cristina Araujo Brinkerhoff, MA (Doctoral RA)
Cristina Brinkerhoff is currently a doctoral candidate with interests in immigrant health disparities, social networks, social support, mental health, immigration policies, and community-based participatory research. Before joining BU as a doctoral student, Cristina worked at the Institute for Community Health as a Research Associate. She also worked as an NIH Research Fellow at BU and UMass Boston on an exploratory study about the implication of transnationalism, culture, and social networks for the health and behavior of Brazilian and Dominican immigrants. She is also currently secretary of the Brazilian Workers Center board. Cristina graduated from UMass Boston with a BA in Sociology in 2011 and a MA in Applied Sociology in 2014.
Inverse Spectroscopic Optical Coherence Tomography for the Detection of Corneal Ultra-Structural Changes in keratoconus and Quantification of Corneal Cross-linking
柯
Meet the Team
 Dr. Hyunjoo Lee is a board-certified ophthalmologist, with fellowship training in cornea, external disease and refractive surgery. As a current attending ophthalmologist at Boston Medical Center and Boston University Eye Associates, and the director of the cornea service, she cares for patients with a variety of corneal and ocular surface conditions, including many patients with keratoconus, a progressive disease of the cornea that can lead to profound vision loss. She is an experienced corneal surgeon, performing corneal cross-linking to treat keratoconus, laser vision correction, cataract surgery, and all types of corneal transplantation.
Dr. Hyunjoo Lee is a board-certified ophthalmologist, with fellowship training in cornea, external disease and refractive surgery. As a current attending ophthalmologist at Boston Medical Center and Boston University Eye Associates, and the director of the cornea service, she cares for patients with a variety of corneal and ocular surface conditions, including many patients with keratoconus, a progressive disease of the cornea that can lead to profound vision loss. She is an experienced corneal surgeon, performing corneal cross-linking to treat keratoconus, laser vision correction, cataract surgery, and all types of corneal transplantation.
博士
Modeling Developmental Alterations Linked to Congenital Hydrocephalus Using Zebrafish
有限公司
 Dr. Arthur Marivin is a Research Assistant Professor in the Department of Biochemistry at Boston University School of Medicine. He received his Ph.D. at the University of Burgundy in Dijon, France. Dr. Marivin’s research focuses on characterizing novel principles of G-protein signaling and defining their role in disease. His previous work as a postdoc in the Garcia-Marcos lab (Department of Biochemistry) uncovered how alterations in G-protein signaling networks cause cancer or birth defects that include congenital hydrocephalus. His research combines dissection of signaling circuits using in vitro biochemistry and synthetic biology tools with disease modeling in vivo using Xenopus and zebrafish embryos.
Dr. Arthur Marivin is a Research Assistant Professor in the Department of Biochemistry at Boston University School of Medicine. He received his Ph.D. at the University of Burgundy in Dijon, France. Dr. Marivin’s research focuses on characterizing novel principles of G-protein signaling and defining their role in disease. His previous work as a postdoc in the Garcia-Marcos lab (Department of Biochemistry) uncovered how alterations in G-protein signaling networks cause cancer or birth defects that include congenital hydrocephalus. His research combines dissection of signaling circuits using in vitro biochemistry and synthetic biology tools with disease modeling in vivo using Xenopus and zebrafish embryos.
Multiomic Phenotyping in Cardiac and Skeletal Muscle in Heart Failure with Preserved Ejection Fraction
他
 Dr. Deepa M. Gopal
Dr. Deepa M. Gopal
Assistant Professor of Medicine, Cardiovascular Division
She is a clinician-investigator whose research focuses on developing a phenotyping strategy to identify early heart failure (HF) with preserved ejection fraction (HFpEF) in individuals with obesity. This strategy will help direct mechanistically-driven therapeutics earlier in an individual’s HF disease trajectory to mitigate risk. This research harnesses her clinical expertise using novel cardiac deformation imaging in echocardiography, invasive and non-invasive cardiopulmonary exercise testing, and circulating/tissue biomarkers as key components of her methodology.
 Dr. Jessica L. Fetterman
Dr. Jessica L. Fetterman
Assistant Professor of Medicine in the Whitaker Cardiovascular Institute
She is a basic and translational scientist in cardiovascular pathophysiology. Her research program utilizes trans-disciplinary approaches to advance their understanding of the role and mechanisms of mitochondrial genetics in cardiovascular pathophysiology. To do so, they evaluate mitochondrial genetics and biology at the population level through genetic epidemiology and systems biology approaches, at the cardiovascular tissue level through rapid autopsy samples and biopsies, and through the creation of cardiovascular cells from human induced pluripotent stem cells. They are creating a number of software packages aimed at enhancing their ability to delineate pathogenic and benign mitochondrial genetic variants and to enable the investigation of mitochondrial-nuclear genetic interactions. Their goal is to identify the mechanisms for how mitochondrial genetic variation contributes to the development of cardiovascular diseases.
Soft Micro Robotic Needle Steering Mechanisms for Interventional Bronchoscopy
Lung cancer remains the leading cause of death from cancer in the U.S. Early diagnosis is
of paramount importance to increase the survival rate of lung cancer and can only be achieved via accurate tissue sampling. This is particularly challenging while navigating in the deep locations of the lungs, where the angles of the various takeoffs of the bronchi can be difficult to access and cancer can remain undetected. Robotic platforms for interventional bronchoscopy, currently available on the market, lack distal dexterity and the capability to achieve sharp turns. This pilot grant will focus on novel robotic actuation mechanisms to steer the tip of biopsy tools and enable tissue sampling in difficult to reach areas of the lung.
Meet the Team
 Dr. Sheila Russo is an Assistant Professor in the Department of Mechanical Engineering and the Division of Materials Science and Engineering at Boston University. She completed her Ph.D. degree at the BioRobotics Institute, Sant’Anna School of Advanced Studies (Italy) and her postdoctoral training at the Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. She is the founder and director of the Material Robotics Laboratory at BU that focuses on design, mechanics, and manufacturing of novel multi-scale and multi-material biomedical robotic systems. Her research interests include medical and surgical robotics, soft robotics, sensing and actuation, meso- and micro-scale manufacturing techniques, and advanced materials.
Dr. Sheila Russo is an Assistant Professor in the Department of Mechanical Engineering and the Division of Materials Science and Engineering at Boston University. She completed her Ph.D. degree at the BioRobotics Institute, Sant’Anna School of Advanced Studies (Italy) and her postdoctoral training at the Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. She is the founder and director of the Material Robotics Laboratory at BU that focuses on design, mechanics, and manufacturing of novel multi-scale and multi-material biomedical robotic systems. Her research interests include medical and surgical robotics, soft robotics, sensing and actuation, meso- and micro-scale manufacturing techniques, and advanced materials.
 Dr. Ehab Billatos is an Assistant Professor of Medicine at Boston University Medical Center specializing in pulmonary and critical care. He serves as the Director of Inpatient Pulmonary Clinical Services, the Director of the Pleural Disease Service, and the Assistant Director of Bronchoscopy at Boston University Medical Center. He has a special interest in advanced bronchoscopic techniques for diagnosis of lung cancer. He also serves as the Principal Investigator for the DECAMP consortium (Detection of Early Cancer Among Military Personnel) which aims to improve the early diagnosis of lung cancer via biomarker validation and development from minimally invasive specimens. This is a multi-center trial sponsored by the Department of Defense, the National Cancer Institute, Johnson & Johnson, and NovartisPharmaceuticals.
Dr. Ehab Billatos is an Assistant Professor of Medicine at Boston University Medical Center specializing in pulmonary and critical care. He serves as the Director of Inpatient Pulmonary Clinical Services, the Director of the Pleural Disease Service, and the Assistant Director of Bronchoscopy at Boston University Medical Center. He has a special interest in advanced bronchoscopic techniques for diagnosis of lung cancer. He also serves as the Principal Investigator for the DECAMP consortium (Detection of Early Cancer Among Military Personnel) which aims to improve the early diagnosis of lung cancer via biomarker validation and development from minimally invasive specimens. This is a multi-center trial sponsored by the Department of Defense, the National Cancer Institute, Johnson & Johnson, and NovartisPharmaceuticals.
2021 Integrated Pilot Grant Awardees
A Patient-Specific Induced Pluripotent Stem Cell (iPSC)-based Organoid Model of Pulmonary Fibrosis
Th

Dr. Konstantinos-Dionysios Alysandratos obtained his medical degree and doctoral degree in Immunopharmacology from the University of Athens in Greece. He completed his internal medicine residency training at the University of Texas Southwestern and Parkland Medical Center in Dallas, TX. Followingly, he pursued clinical fellowships in Sleep Medicine and Pulmonary/Critical Care Medicine at Boston University Medical School and Boston Medical Center. During his fellowship training, he joined Dr. Darrell Kotton’s laboratory at the Center for Regenerative Medicine (CReM) of Boston University and Boston Medical Center. He is currently an Assistant Professor of Medicine at Boston University School of Medicine.
His current research aims to expand our scant knowledge of the pathogenesis of common lung diseases such as interstitial lung disease (ILD) and specifically the role of alveolar type 2 (AT2) cells at the inception of disease. Under the mentorship of Dr. Darrell Kotton, he has developed an in vitro model system that permits investigation of epithelial-intrinsic events that lead to AT2 cell dysfunction over time using patient-derived cells that carry a disease-associated variant, SFTPCI73T, known to be expressed exclusively in AT2 cells.
Dr. Alysandratos has received several honors and awards throughout his career including from the American Thoracic Society and the American Lung Association. While in residency training, he was voted as an honorary member of the Alpha Omega Alpha Medical Honor Society by the university’s academic community. Most recently, Dr. Alysandratos received the Pulmonary Fibrosis Foundation I.M. Rosenzweig Junior Investigator Award for his innovative research on stem cell-based models of pulmonary fibrosis.
Culturally-Adapted Motivational Interviewing and Contingency Management for Reducing Illicit Stimulant Use in Black and Latin People
This CTSI pilot study is preliminary work in attempting to combine three approaches to treating stimulant use disorder in Black and Latinx people: 1) culturally adapted motivational interviewing; 2) recovery coaching; and 3) contingency management. We will first conduct focus groups in Black and Latinx people who use stimulants, in order to inform the adaptation of an existing motivational interviewing intervention. We will then pilot test this adapted intervention, which will be delivered by recovery coaches, in a clinic that provides contingency management treatment for stimulant use.
Meet the Team
 Dr. Tae Woo (Ted) Park is an Assistant Professor of Psychiatry at Boston University School of Medicine. He is an addiction psychiatrist and researcher. He has a NIDA K23 award that aims to reduce benzodiazepine use in patients who receive opioid agonist treatment. His clinical focus is on the treatment of addiction and its psychiatric comorbidities. He is the medical director of the ABOVE program at Boston Medical Center, a program that focuses on treating co-occurring opioid use and mental disorders, and a consultant for BMC’s CATALYST program for transitional age youth with substance use disorders. In addition, he is active in the education of residents and addiction psychiatry fellows at Boston University/Boston Medical Center. He completed his psychiatry training at Western Psychiatric Institute and Clinic in Pittsburgh, PA, and his postdoctoral research at the Boston VA and Boston University School of Medicine.
Dr. Tae Woo (Ted) Park is an Assistant Professor of Psychiatry at Boston University School of Medicine. He is an addiction psychiatrist and researcher. He has a NIDA K23 award that aims to reduce benzodiazepine use in patients who receive opioid agonist treatment. His clinical focus is on the treatment of addiction and its psychiatric comorbidities. He is the medical director of the ABOVE program at Boston Medical Center, a program that focuses on treating co-occurring opioid use and mental disorders, and a consultant for BMC’s CATALYST program for transitional age youth with substance use disorders. In addition, he is active in the education of residents and addiction psychiatry fellows at Boston University/Boston Medical Center. He completed his psychiatry training at Western Psychiatric Institute and Clinic in Pittsburgh, PA, and his postdoctoral research at the Boston VA and Boston University School of Medicine.

博士
 Dr. Christina S. Lee is an associate professor in the School of Social Work at Boston University (BUSSW) and a faculty affiliate at BU’s Center for Antiracist Research. Her research bridges the areas of intervention science, addiction psychology, and health disparities. By focusing on the effects of social and environmental stressors, Dr. Lee has become an influential voice in efforts to reduce risky health behaviors among diverse, understudied groups. She is PI and co-PI on NIH-funded addiction treatment research, affiliate training faculty at the Center for Alcohol and Addiction Studies at Brown University, research core director at the BUSSW Center for Innovation in Social Work and Health, and mentors graduate and postdoctoral scholars from diverse racial-ethnic groups. Dr. Lee is a member of the editorial board of the Journal of Consulting and Clinical Psychology.
Dr. Christina S. Lee is an associate professor in the School of Social Work at Boston University (BUSSW) and a faculty affiliate at BU’s Center for Antiracist Research. Her research bridges the areas of intervention science, addiction psychology, and health disparities. By focusing on the effects of social and environmental stressors, Dr. Lee has become an influential voice in efforts to reduce risky health behaviors among diverse, understudied groups. She is PI and co-PI on NIH-funded addiction treatment research, affiliate training faculty at the Center for Alcohol and Addiction Studies at Brown University, research core director at the BUSSW Center for Innovation in Social Work and Health, and mentors graduate and postdoctoral scholars from diverse racial-ethnic groups. Dr. Lee is a member of the editorial board of the Journal of Consulting and Clinical Psychology.
Epidemiology COVID-19 Response Corps
In response to the COVID-19 pandemic, Drs. Ellie Murray and Jennifer Weuve created the Epidemiology COVID-19 Response Corps (or COVID Corps for short), which brought together over 150 students, faculty, and alumni to help fill gaps in COVID research and develop messaging tools to help people understand how to stay safe. Our CTSI funded project will conduct an impact evaluation of the COVID Corps so that we can understand what worked well, what didn’t work as well, and what we can do better going forward during this pandemic and in future public health crises.

博士
 Dr. Jennifer Weuve is an associate professor of epidemiology at the Boston University School of Public Health. In her research, she seeks to identify causes of cognitive decline and dementia, particularly environmental causes. Undergirding this research is her dedication to aligning epidemiologic methods to solving public health problems. She is the PI or co-investigator of several NIH-supported investigations and projects in all of these realms. During the COVID-19 pandemic and with Dr. Ellie Murray, she co-founded the BUSPH Epidemiology COVID-19 Response Corps, which engages with students, faculty, and other BU community members to address important epidemiologic issues related to the COVID-19 pandemic, including research, communication, policy, and best practices. Dr. Weuve earned her master’s in public health degree from the University of Minnesota and her doctoral degree from the Harvard TH Chan School of Public Health.
Dr. Jennifer Weuve is an associate professor of epidemiology at the Boston University School of Public Health. In her research, she seeks to identify causes of cognitive decline and dementia, particularly environmental causes. Undergirding this research is her dedication to aligning epidemiologic methods to solving public health problems. She is the PI or co-investigator of several NIH-supported investigations and projects in all of these realms. During the COVID-19 pandemic and with Dr. Ellie Murray, she co-founded the BUSPH Epidemiology COVID-19 Response Corps, which engages with students, faculty, and other BU community members to address important epidemiologic issues related to the COVID-19 pandemic, including research, communication, policy, and best practices. Dr. Weuve earned her master’s in public health degree from the University of Minnesota and her doctoral degree from the Harvard TH Chan School of Public Health.
Generation of a Conditional Mouse Strain for GNB2 to Investigate Legionellosis
In the current CTSI pilot grant, Dr. Bosmann and his co-workers aim to generate a novel floxed mouse strain for tissue-specific gene deletion of the G-protein subunit, GNB2. The conditional knockout mice will later be used to study the role of GNB2 in myeloid cells during Legionella infection of the lung.
 Dr. Markus Bosmann is an Associate Professor in the Pulmonary Center, Department of Medicine holding secondary appointments in the Department of Pathology & Laboratory Medicine, BUSM, and the National Emerging Infectious Diseases Laboratories (NEIDL). He graduated as a Doctor of Medicine, with a summa cum laude honor thesis, from the Johann Wolfgang Goethe University, Frankfurt, Germany. He continued his career with residency training in medicine, laboratory medicine, and a research postdoctoral fellowship at the University of Michigan, Ann Arbor, followed by a junior faculty appointment at the Johannes Gutenberg University, Mainz, Germany. His research interests are focused on the cellular and molecular pathogenesis of infection-associated inflammation. The work in his multidisciplinary, diverse research team is centered around understanding the host response to microbial pathogens in the context of lung injury, pneumonia, and sepsis. Dr. Bosmann is also the director of the newly established Affinity Research Collaborative (ARC) named Respiratory Viruses: A Focus on COVID-19. He was awarded the Hugo-Schottmüller prize of the German Sepsis Society in 2020.
Dr. Markus Bosmann is an Associate Professor in the Pulmonary Center, Department of Medicine holding secondary appointments in the Department of Pathology & Laboratory Medicine, BUSM, and the National Emerging Infectious Diseases Laboratories (NEIDL). He graduated as a Doctor of Medicine, with a summa cum laude honor thesis, from the Johann Wolfgang Goethe University, Frankfurt, Germany. He continued his career with residency training in medicine, laboratory medicine, and a research postdoctoral fellowship at the University of Michigan, Ann Arbor, followed by a junior faculty appointment at the Johannes Gutenberg University, Mainz, Germany. His research interests are focused on the cellular and molecular pathogenesis of infection-associated inflammation. The work in his multidisciplinary, diverse research team is centered around understanding the host response to microbial pathogens in the context of lung injury, pneumonia, and sepsis. Dr. Bosmann is also the director of the newly established Affinity Research Collaborative (ARC) named Respiratory Viruses: A Focus on COVID-19. He was awarded the Hugo-Schottmüller prize of the German Sepsis Society in 2020.
HIV-1 RNA Modification as a Driver of Innate Immune Activation
嗨
Meet the Team
 Dr. Hisashi Akiyama received his Ph.D. from Kyoto University, Japan. He performed his postdoctoral research at the University of Heidelberg in Germany where he worked on cellular and molecular mechanisms of HIV replication. He is currently a Research Assistant Professor in the Department of Microbiology at Boston University School of Medicine. His research goal is to understand the pathogenesis of HIV. In particular, he is interested in the role of myeloid cells in the establishment and dissemination of HIV infection and mechanisms of virus evasion from innate and adaptive host immune responses.
Dr. Hisashi Akiyama received his Ph.D. from Kyoto University, Japan. He performed his postdoctoral research at the University of Heidelberg in Germany where he worked on cellular and molecular mechanisms of HIV replication. He is currently a Research Assistant Professor in the Department of Microbiology at Boston University School of Medicine. His research goal is to understand the pathogenesis of HIV. In particular, he is interested in the role of myeloid cells in the establishment and dissemination of HIV infection and mechanisms of virus evasion from innate and adaptive host immune responses.
 Dr. Daniel Cifuentes is an Assistant Professor in the Department of Biochemistry at the Boston University School of Medicine. Dr. Cifuentes received his Ph.D. from the University of Barcelona, Spain, for his studies in metabolic regulation. He completed his postdoctoral training in the Department of Genetics at Yale University School of Medicine, where he specialized in microRNA biology and vertebrate embryogenesis. His research goals at Boston University combine high-throughput genetic, genomic, and proteomic approaches to address fundamental questions of RNA regulation in viral and vertebrate systems.
Dr. Daniel Cifuentes is an Assistant Professor in the Department of Biochemistry at the Boston University School of Medicine. Dr. Cifuentes received his Ph.D. from the University of Barcelona, Spain, for his studies in metabolic regulation. He completed his postdoctoral training in the Department of Genetics at Yale University School of Medicine, where he specialized in microRNA biology and vertebrate embryogenesis. His research goals at Boston University combine high-throughput genetic, genomic, and proteomic approaches to address fundamental questions of RNA regulation in viral and vertebrate systems.
Highly Multiplexed Immunophenotyping of Aggressive Histologic Patterns of Early-Stage Lung Adenocarcinomas
陆
Meet the Team
 Dr. Eric Burks is a Clinical Associate Professor of Pathology at the Boston University School of Medicine and the Medical Director for Hematopathology and Immunohistochemistry at Boston Medical Center. After graduating from medical school at the University of New Mexico School of Medicine, he had two fellowships: one in Surgical Pathology at Harvard Medical School and another in Hematopathology at Johns Hopkins Medical Institution. He joined the faculty at Boston University in 2018 where he brought extensive experience as a lecturer, mentor, and physician with a particular interest in lung cancer pathology. In his role as Medical Director for Hematopathology and Immunohistochemistry Eric has established a repository of well-annotated lung cancer samples that will be immune profiled as part of these studies to support additional funding opportunities for ongoing studies to establish biomarkers to better detect aggressive lung cancers.
Dr. Eric Burks is a Clinical Associate Professor of Pathology at the Boston University School of Medicine and the Medical Director for Hematopathology and Immunohistochemistry at Boston Medical Center. After graduating from medical school at the University of New Mexico School of Medicine, he had two fellowships: one in Surgical Pathology at Harvard Medical School and another in Hematopathology at Johns Hopkins Medical Institution. He joined the faculty at Boston University in 2018 where he brought extensive experience as a lecturer, mentor, and physician with a particular interest in lung cancer pathology. In his role as Medical Director for Hematopathology and Immunohistochemistry Eric has established a repository of well-annotated lung cancer samples that will be immune profiled as part of these studies to support additional funding opportunities for ongoing studies to establish biomarkers to better detect aggressive lung cancers.
Identifying Coding and Non-Coding Genomic Alterations Associated with Aggressive Prostate Cancer in African American Men
In collaboration with Drs. Joshua Campbell and Rachel Flynn, the overall goal of this pilot project is to identify coding and non-coding genomic alterations in the racially and socioeconomically diverse patient population surgically treated for prostate cancer at Boston Medical Cancer. In particular, we aim to identify a genomic signature associated with more aggressive disease in African American men, as well as establishing a pipeline to integrate these genomic data with other biomarker measurements (e.g. presence of ctDNA, microbiome signatures) and clinical outcomes in a larger cohort of men to create a comprehensive snapshot of these patients at the time of diagnosis and subsequently following treatment.
Meet the Team
 Dr. Christopher Heaphy is a member of the Boston University-Boston Medical Center Cancer Center and is an Assistant Professor of Medicine and Pathology & Laboratory Medicine. His research program uses a combination of tissue-based, cell-based, and molecular approaches to study genomic alterations as it relates to cancer initiation and progression across a wide range of cancer types. In addition, his laboratory focuses on how the detection of these alterations may be readily translated into accurately predicting cancer risk, early detection, prognosis, and potential response to targeted therapies.
Dr. Christopher Heaphy is a member of the Boston University-Boston Medical Center Cancer Center and is an Assistant Professor of Medicine and Pathology & Laboratory Medicine. His research program uses a combination of tissue-based, cell-based, and molecular approaches to study genomic alterations as it relates to cancer initiation and progression across a wide range of cancer types. In addition, his laboratory focuses on how the detection of these alterations may be readily translated into accurately predicting cancer risk, early detection, prognosis, and potential response to targeted therapies.

Dr. Joshua D. Campbell received his Ph.D. in Bioinformatics from Boston University. He performed his postdoctoral research at the Dana-Farber Cancer Institute and the Broad Institute of Harvard and MIT where he worked with The Cancer Genome Atlas (TCGA) to identify novel mutational drivers of lung cancer. He is currently an assistant professor in the Department of Medicine at Boston University School of Medicine where he utilizes genomic data to uncover biological mechanisms that may contribute to cancer disparities.
 Dr. Rachel Flynn received her Ph.D. in Cancer Biology from the University of Massachusetts Medical School and conducted her Postdoctoral Fellowship in Molecular Oncology at the Massachusetts General Hospital at Harvard Medical School. Dr. Flynn is currently an Associate Professor in the Departments of Pharmacology & Experimental Therapeutics, and Medicine where the focus of her lab is to define the mechanisms regulating mammalian telomere maintenance and to understand how defects in this process contribute to tumorigenesis. She hopes these studies will allow her lab to gain the mechanistic insight necessary to identify novel targets and/or strategies in the treatment of cancer.
Dr. Rachel Flynn received her Ph.D. in Cancer Biology from the University of Massachusetts Medical School and conducted her Postdoctoral Fellowship in Molecular Oncology at the Massachusetts General Hospital at Harvard Medical School. Dr. Flynn is currently an Associate Professor in the Departments of Pharmacology & Experimental Therapeutics, and Medicine where the focus of her lab is to define the mechanisms regulating mammalian telomere maintenance and to understand how defects in this process contribute to tumorigenesis. She hopes these studies will allow her lab to gain the mechanistic insight necessary to identify novel targets and/or strategies in the treatment of cancer.
Liver-dependent Lung Remodeling and Pneumonia Susceptibility During Sepsis
It has long been known that systemic inflammatory events, such as those inherent to sepsis, tremendously predispose patients to hospital-acquired lung infections. Yet, the biological pathways influencing lung immunity during sepsis are poorly understood. Dr. Lee Quinton’s research has now established that liver responses to systemic inflammation calibrate the immunological tone of lung tissue, increasing its capacity to respond to subsequent infections. This study will leverage liver-specific mutant mouse models and single-cell sequencing to delineate cell-specific changes within the lung that rely on intact liver function, pursuing the hypothesis that during sepsis, liver activity dictates the transcriptional fingerprint of lung cells, including alveolar macrophages, to limit pneumonia susceptibility.
 Dr. Lee Quinton is an Associate Professor of Medicine, Microbiology, and Pathology. He joined the faculty in the Pulmonary Center in 2008 after completing his post-doctoral fellowship at the Harvard School of Public Health. Dr. Quinton’s research program focuses on molecular mechanisms governing immunity and tissue protection during pneumonia. In particular, his laboratory has identified liver activation as a conduit through which early response cytokines promote local and systemic defense in response to lung infections. Additional investigations in Dr. Quinton’s laboratory are focused on deciphering signals controlling epithelial barrier integrity in the pneumonic lung, with the specific goal of determining when, where, and how lung cells collaborate to limit inflammatory injury.
Dr. Lee Quinton is an Associate Professor of Medicine, Microbiology, and Pathology. He joined the faculty in the Pulmonary Center in 2008 after completing his post-doctoral fellowship at the Harvard School of Public Health. Dr. Quinton’s research program focuses on molecular mechanisms governing immunity and tissue protection during pneumonia. In particular, his laboratory has identified liver activation as a conduit through which early response cytokines promote local and systemic defense in response to lung infections. Additional investigations in Dr. Quinton’s laboratory are focused on deciphering signals controlling epithelial barrier integrity in the pneumonic lung, with the specific goal of determining when, where, and how lung cells collaborate to limit inflammatory injury.
Modeling the Anemia Elicited by Chronic Kidney Disease in Zebrafish
The goal of this research is to uncover how the interplay between microRNAs and uremic solutes orchestrate the responsiveness to erythropoiesis-stimulating agents (ESA) in chronic kidney disease (CKD). To this end, this project will model the anemia of CKD using zebrafish embryos as a model system. Preliminary data suggest a protective role of miR-451 in front of the oxidative stress induced by the uremic milieu. The findings from this project will have the potential to be developed into biomarkers and drug targets to enhance the precision of ESA therapeutics.
At the intersection of uremic toxicity and microRNAs, this proposal is innovative because it probes, for the first time, the effect of a set of uremic solutes on microRNA biogenesis, examines zebrafish as a model of uremic toxicity and the mechanism of ESA-responsiveness in end-stage renal disease patients. The novelty of the proposal is driven by an interdisciplinary team experienced in microRNAs and zebrafish (Dr. Cifuentes), and uremic toxicity (Dr. Chitalia).
Meet the Team
 Dr. Daniel Cifuentes is an Assistant Professor in the Department of Biochemistry at the Boston University School of Medicine. Dr. Cifuentes received his Ph.D. from the University of Barcelona, Spain, for his studies in metabolic regulation. He completed his postdoctoral training in the Department of Genetics at Yale University School of Medicine, where he specialized in microRNA biology and vertebrate embryogenesis. His research goals at Boston University combine high-throughput genetic, genomic, and proteomic approaches to address fundamental questions of RNA regulation in viral and vertebrate systems.
Dr. Daniel Cifuentes is an Assistant Professor in the Department of Biochemistry at the Boston University School of Medicine. Dr. Cifuentes received his Ph.D. from the University of Barcelona, Spain, for his studies in metabolic regulation. He completed his postdoctoral training in the Department of Genetics at Yale University School of Medicine, where he specialized in microRNA biology and vertebrate embryogenesis. His research goals at Boston University combine high-throughput genetic, genomic, and proteomic approaches to address fundamental questions of RNA regulation in viral and vertebrate systems.
 Dr. Vipul Chitalia is an Associate Professor of Medicine at Boston University School of Medicine and an attending nephrologist covering renal consults, dialysis, and kidney transplant in-patient services as well as an affiliation to Harvard-MIT Division of Science and Technology, MIT. Teaching is his passion and he considers it an honor to be able to contribute to the training of the next generation of physicians, scientists, and physician-scientists. He strongly believes that a true mentor is one who inspires passion from within and empowers a mentee to find his or her own path of success.
Dr. Vipul Chitalia is an Associate Professor of Medicine at Boston University School of Medicine and an attending nephrologist covering renal consults, dialysis, and kidney transplant in-patient services as well as an affiliation to Harvard-MIT Division of Science and Technology, MIT. Teaching is his passion and he considers it an honor to be able to contribute to the training of the next generation of physicians, scientists, and physician-scientists. He strongly believes that a true mentor is one who inspires passion from within and empowers a mentee to find his or her own path of success.
Multimodal Microscopic Characterization of Novel Humanized Mouse Models of COVID-19
即时通讯

Dr. Nicholas Crossland is a veterinary pathologist, Assistant Professor in the Department of Pathology, and the director of the NEIDL’s Comparative Pathology Laboratory (NCPL) core. His team provides pathology expertise to NEIDL investigators and their respective laboratories working in BSL-3 and BSL-4. His research interest is in the systematic multimodal microscopic characterization of animal models to better define pathogenic mechanisms of emerging infectious diseases. He also finds fulfillment in utilizing his expertise to evaluate the efficacy of vaccines and therapeutics in preclinical models as a means to prevent and/or alleviate human suffering.
Non-invasive Blood Flow to Predict Recovery from Coma in Brain Injured Patients
We aim to determine the effect of cerebral blood flow on coma recovery, testing the hypothesis that non-invasive cerebral blood flow measurements following acute brain injury can predict recovery. We will use a novel non-invasive monitor to determine CBF, continuously following admission in stroke, traumatic brain injury (TBI), and post-cardiac arrest comatose patients. We will also evaluate the effect of skin color on non-invasive optical blood flow measures, with the understanding that skin color may introduce a correctable bias in cerebral blood flow measurements in awake and comatose brain patients, important to our diverse populations at BU/BMC.

Dr. David Greer is Professor and Chair of the Department of Neurology at Boston University School of Medicine and the Richard B. Slifka Chief of Neurology at Boston Medical Center.
Dr. Greer is the editor-in-chief of Seminars in Neurology, and the immediate past editor-in-chief for Neurocritical Care ON CALL. He has authored more than 250 peer-reviewed manuscripts, reviews, chapters, guidelines, and books.
His research interests include predicting recovery from coma after cardiac arrest, brain death, and multiple stroke-related topics, including acute stroke treatment, temperature modulation, and stroke prevention.
 Dr. David Chung is a neurointensivist and translational neuroscientist in the Department of Neurology. He completed his MD/PhD and Neurology residency at Columbia University and a fellowship in Neurocritical Care at Harvard-MGB. Dr. Chung is an expert in cerebral blood flow and is dedicated to finding ways to improve long-term outcomes in patients with acute brain injury.
Dr. David Chung is a neurointensivist and translational neuroscientist in the Department of Neurology. He completed his MD/PhD and Neurology residency at Columbia University and a fellowship in Neurocritical Care at Harvard-MGB. Dr. Chung is an expert in cerebral blood flow and is dedicated to finding ways to improve long-term outcomes in patients with acute brain injury.

Dr. David Boas is a professor in Biomedical Engineering. His research areas of interest are: Neurophotonics, Biomedical Optics, Oxygen delivery and consumption, Neuro-vascular coupling, Physiological Modeling
Patient Perspectives on Disrespect and Abuse in Maternity Care
交货
 Dr. Rachel Cannon is an Assistant Professor in Obstetrics and Gynecology at Boston University School of Medicine and an attending physician in the department of Obstetrics and Gynecology at Boston Medical Center. She provides full-scope OBGYN care and specializes in complex contraception and abortion. Her academic research interests include contraceptive counseling, reproductive justice, and health equity. She co-chairs the Health Equity Committee in the Department of OBGYN.
Dr. Rachel Cannon is an Assistant Professor in Obstetrics and Gynecology at Boston University School of Medicine and an attending physician in the department of Obstetrics and Gynecology at Boston Medical Center. She provides full-scope OBGYN care and specializes in complex contraception and abortion. Her academic research interests include contraceptive counseling, reproductive justice, and health equity. She co-chairs the Health Equity Committee in the Department of OBGYN.
Dr. Cannon completed an undergraduate degree in Human Physiology at Boston University. She then completed her medical degree at the University of Massachusetts followed by a residency in OBGYN at Northwestern University. She specialized in abortion and complex contraception by completing a fellowship in Family Planning at Boston Medical Center. While in fellowship, she earned a Masters of Science in Health Services Research through Boston University School of Public Health. She advises the Boston University School of Medicine ACOG OBGYN Interest Group. Dr. Cannon serves as Ryan Program Director in the Department of OBGYN residency program and is responsible for the family planning curriculum for OBGYN residents.
 Dr. Katharine Hutchinson is an Assistant Professor in Obstetrics and Gynecology at BUSM and an attending midwife at BMC. She is the Co-Director of Advocacy Initiatives for the Department and provides full-scope midwifery care to clients at BMC. Her research interests include Respectful Maternity Care and patient experiences of birth during COVID.
Dr. Katharine Hutchinson is an Assistant Professor in Obstetrics and Gynecology at BUSM and an attending midwife at BMC. She is the Co-Director of Advocacy Initiatives for the Department and provides full-scope midwifery care to clients at BMC. Her research interests include Respectful Maternity Care and patient experiences of birth during COVID.
Dr. Hutchinson completed her undergraduate degree at Swarthmore College, followed by her midwifery training at Yale University, and her doctorate at BUSPH. Her dissertation examined access to emergency obstetric care for preeclamptic and eclamptic patients in Port au Prince, Haiti.
Prediction of Knee Pain Using Ultrasound Imaging and Machine Learning
This project (in collaboration with Dr. Juan-Pablo Lopez-Zertuche Ortiz, Dr. Eugene Kissin, and Dr. David Felson) proposes to develop advanced machine learning approaches that can process ultrasound images to predict knee pain.
Meet the Team
 Dr. Vijaya Kolachalama is an Assistant Professor within the Department of Medicine, Boston University School of Medicine and the Department of Computer Science, Boston University, and a founding member of the Faculty of Computing & Data Sciences at Boston University. Research in his group is focused on developing advanced machine learning algorithms that have diagnostic relevance. His laboratory is funded by multiple awards from the National Institutes of Health, American Heart Association, private foundations, and the pharmaceutical industry. His course entitled Machine Learning for Biomedical Applications attracts a diverse set of individuals with little to no background in computer science.
Dr. Vijaya Kolachalama is an Assistant Professor within the Department of Medicine, Boston University School of Medicine and the Department of Computer Science, Boston University, and a founding member of the Faculty of Computing & Data Sciences at Boston University. Research in his group is focused on developing advanced machine learning algorithms that have diagnostic relevance. His laboratory is funded by multiple awards from the National Institutes of Health, American Heart Association, private foundations, and the pharmaceutical industry. His course entitled Machine Learning for Biomedical Applications attracts a diverse set of individuals with little to no background in computer science.
I serve as the program director for the rheumatology fellowship program and have a shared focus in medical education and in musculoskeletal ultrasound development. I helped found and lead the training program for USSONAR, the preeminent group for musculoskeletal ultrasound education in North America. I was selected to the American College of Rheumatology (ACR) Core Expert Panel for appropriateness criteria for musculoskeletal ultrasound use in rheumatology as well as the ACR musculoskeletal ultrasound task force and RhMSUS Development Project for musculoskeletal ultrasound certification. In addition, I am responsible for medical student and resident rheumatology rotations at Boston University Medical Center.
Role of ZEB2 in Pericytes Development and Renal Fibrosis
The goal of this pilot grant is to elucidate the role of ZEB2 in kidney fibrosis. ZEB2 is a SMAD-interacting transcriptional factor and is expressed in Foxd1+ stromal cells that are progenitors for pericytes and fibroblasts during kidney development. Pericytes and fibroblasts are the key cell types contributing to the myofibroblasts during kidney fibrosis. We will analyze the histopathological changes in the kidneys of Zeb2 stromal-specific conditional knockout mice (Zeb2flox/flox; Foxd1Cre+) which developed kidney fibrosis. The outcome of this project will help understand the cellular and molecular mechanisms of kidney fibrosis and pericyte/fibroblast development.
 Dr. Sudhir Kumar is an assistant professor of medicine in the Nephrology Section, Department of Medicine at Boston University School of Medicine. His research work focuses on Slit-Robo signaling in kidney diseases and podocyte biology and ZEB2 signaling in kidney development and diseases using animal models. Dr. Kumar received his Ph.D. from Ludwig Maximilians University Munich, Germany, and completed his postdoctoral training in Dr. Weining Lu’s lab, Nephrology Section at Boston University School of Medicine.
Dr. Sudhir Kumar is an assistant professor of medicine in the Nephrology Section, Department of Medicine at Boston University School of Medicine. His research work focuses on Slit-Robo signaling in kidney diseases and podocyte biology and ZEB2 signaling in kidney development and diseases using animal models. Dr. Kumar received his Ph.D. from Ludwig Maximilians University Munich, Germany, and completed his postdoctoral training in Dr. Weining Lu’s lab, Nephrology Section at Boston University School of Medicine.
SARS-CoV-2 Diversity and Transmission Among Healthcare Personnel
Healthcare personnel (HCP) have been disproportionately affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), however little is currently known about where and under what conditions SARS-CoV-2 transmission occurs in clinical settings. This study will combine viral whole-genome sequencing with rich epidemiological and contact tracing data on a unique cohort of healthcare workers and nosocomial patient cases spanning the initial COVID-19 surge in Boston. With a team of experts from Boston Medical Center, Boston University School of Public Health, and the Boston University National Emerging Infectious Diseases Laboratory, we aim to uncover how SARS-CoV-2 transmission in the hospital setting was successfully extinguished and identify circumstances where outbreaks persisted. A better understanding of SARS-CoV-2 transmission dynamics among healthcare workers and their patients will provide essential information for limiting hospital transmission in the ongoing pandemic.
Meet the Team
 Dr. Tara Bouton is an assistant professor of medicine in the Section of Infectious Disease at Boston University School of Medicine. Her primary research interests are in the clinical and molecular epidemiology and genomics of drug resistance of tuberculosis and the impact of HIV co-infection. However, over the last year, she has served as co-investigator in two COVID-19 clinical trials and helped to develop a large healthcare worker SARS-CoV-2 cohort study using viral whole-genome sequencing to understand nosocomial transmission dynamics. She is currently PI on a prospective effort to bring near real-time sequencing to infection control at BMC.
Dr. Tara Bouton is an assistant professor of medicine in the Section of Infectious Disease at Boston University School of Medicine. Her primary research interests are in the clinical and molecular epidemiology and genomics of drug resistance of tuberculosis and the impact of HIV co-infection. However, over the last year, she has served as co-investigator in two COVID-19 clinical trials and helped to develop a large healthcare worker SARS-CoV-2 cohort study using viral whole-genome sequencing to understand nosocomial transmission dynamics. She is currently PI on a prospective effort to bring near real-time sequencing to infection control at BMC.
 Dr. John Connor is an Associate Professor of Microbiology at Boston University School of Medicine and an investigator at the National Emerging Infectious Diseases Laboratory. He leads a team of researchers that are studying the virus-host interaction through a variety of strategies including photonics, molecular virology, cell biology, and different omics approaches. These efforts include discreet programs in developing antiviral drugs, light-based diagnostics, and predictive/prognostic biomarker ID to help identify and treat viral disease. These studies have in the past focused on diseases like Ebola, Marburg, and Lassa Fever. During the COVID-19 pandemic, his laboratory has been heavily involved in mapping SARS-CoV-2 transmission through populations by analyzing the virus genome from patient samples and tracking its evolution.
Dr. John Connor is an Associate Professor of Microbiology at Boston University School of Medicine and an investigator at the National Emerging Infectious Diseases Laboratory. He leads a team of researchers that are studying the virus-host interaction through a variety of strategies including photonics, molecular virology, cell biology, and different omics approaches. These efforts include discreet programs in developing antiviral drugs, light-based diagnostics, and predictive/prognostic biomarker ID to help identify and treat viral disease. These studies have in the past focused on diseases like Ebola, Marburg, and Lassa Fever. During the COVID-19 pandemic, his laboratory has been heavily involved in mapping SARS-CoV-2 transmission through populations by analyzing the virus genome from patient samples and tracking its evolution.
Soft Robotic Technologies Enabling Safe Laparoscopic Bowel Manipulation
拉
 Dr. Tommaso Ranzani is an Assistant Professor in the Department of Mechanical Engineering, Biomedical Engineering, and in the Division of Materials Science and Engineering at Boston University. He received a Bachelor’s and Master’s degree in Biomedical Engineering from the University of Pisa, Italy. He did his Ph.D. at the BioRobotics Institute of the Sant’Anna School of Advanced Studies, and he joined the Wyss Institute for Biologically Inspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences as a postdoctoral fellow in 2014.
Dr. Tommaso Ranzani is an Assistant Professor in the Department of Mechanical Engineering, Biomedical Engineering, and in the Division of Materials Science and Engineering at Boston University. He received a Bachelor’s and Master’s degree in Biomedical Engineering from the University of Pisa, Italy. He did his Ph.D. at the BioRobotics Institute of the Sant’Anna School of Advanced Studies, and he joined the Wyss Institute for Biologically Inspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences as a postdoctoral fellow in 2014.
At Boston University, he founded the Morphable Biorobotics Lab. The lab focuses on expanding the potential of soft robots across different scales to develop novel reconfigurable soft-bodied systems with applications ranging from environmental exploration to assistive and soft surgical robots.

Dr. Donald T. Hess is a graduate of Williams College in Williamstown, MA, and received his medical degree from the University of Rochester School of Medicine and Dentistry in Rochester, NY. He completed a residency in general surgery at Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, and a research fellowship in Surgical Oncology at New England Deaconess Hospital in Boston, MA. He started at Boston University Medical Center after working for the US Air Force as an Assistant Professor of Surgery stationed at Wright-Patterson Air Force Base (WPAFB) in Ohio.
His clinical practice is devoted to minimally invasive and bariatric surgery. He has tremendous surgical expertise in laparoscopic bariatric surgery (sleeve gastrectomy and gastric bypass) and revisional bariatric surgery. He also has an interest in minimally invasive surgery, robotic surgery, surgery for disease of the stomach and esophagus, intestinal surgery, single-site surgery, complex hernia surgery, and general surgery. He is also the program director of the General Surgery residency and is heavily involved in surgical education.
Dr. Hess is involved in many research protocols involving metabolic and cardiovascular disease in patients undergoing bariatric surgery. He is a member of the Association of Metabolic and Bariatric Surgeons, the Society of American Gastrointestinal and Endoscopic Surgeons, a fellow of the American College of Surgeons (ACS). He has been recognized by Boston Magazine’s “Top Docs” issue for being “top” in his respective field since 2009 and was a 2013 recipient of the Boston University School of Medicine Evans Center Collaborator of the Year Award.
Validating the Role of Extracellular Matrix Molecules in Parkinson’s Disease Using Mass Spectrometry Glycomics and Proteomics
Br
我们
We will conduct this work at the BU Center for Biomedical Mass Spectrometry, where Dr. Sethi (PI of the project) works as an Instructor. The biospecimens will be provided by Dr. Thor D. Stein, a neuropathologist at the BU-VA-CLF Brain bank, who will serve as a Co-PI on the project.
 Dr. Manveen K. Sethi serves as Instructor of Biochemistry at the Boston University School of Medicine, USA. She did her Ph.D. at Macquarie University (MQ), Australia, under the primary supervision of Dr. Morten Thaysen-Andersen, where she utilized mass spectrometry proteomics and glycomics analysis to understand underlying molecular mechanisms in colorectal cancer. After her Ph.D., she joined Boston University School of Medicine (BUSM), USA, as a postdoctoral associate under Prof. Joseph Zaia, where she is currently employed in a research faculty-track position of Instructor. Her research work involves identifying and characterizing biomolecules such as proteins and glycans using mass spectrometry techniques and utilizing this information to understand biomolecular deregulation in human diseases, such as cancer and Alzheimer’s disease. Recently, she received a Bright Focus Foundation fellowship award to investigate extracellular matrix changes in Alzheimer’s disease.
Dr. Manveen K. Sethi serves as Instructor of Biochemistry at the Boston University School of Medicine, USA. She did her Ph.D. at Macquarie University (MQ), Australia, under the primary supervision of Dr. Morten Thaysen-Andersen, where she utilized mass spectrometry proteomics and glycomics analysis to understand underlying molecular mechanisms in colorectal cancer. After her Ph.D., she joined Boston University School of Medicine (BUSM), USA, as a postdoctoral associate under Prof. Joseph Zaia, where she is currently employed in a research faculty-track position of Instructor. Her research work involves identifying and characterizing biomolecules such as proteins and glycans using mass spectrometry techniques and utilizing this information to understand biomolecular deregulation in human diseases, such as cancer and Alzheimer’s disease. Recently, she received a Bright Focus Foundation fellowship award to investigate extracellular matrix changes in Alzheimer’s disease.
2020 Integrated Pilot Grant Awardees
Peer Recovery Coaching for HCV and Opioid Use Disorder Treatment
The U.S. opioid epidemic is associated with a surge in hepatitis C virus (HCV) infections among persons who inject drugs. Despite the availability of curative therapy, treatment uptake remains low in this group. The goal of this study is to determine the feasibility and acceptability of a peer intervention to improve linkage to HCV care, treatment initiation, and cure among individuals with a history of opioid use disorder. Our findings will serve as pilot data for a planned NIH R01 proposal.
 Sabrina A. Assoumou, MD, MPH, is Assistant Professor of Medicine at Boston University School of Medicine and an attending physician in the section of Infectious Diseases at Boston Medical Center. She is a clinician-investigator who provides care to human immunodeficiency virus (HIV)-infected patients at the BUSM/BMC’s Centers for Infectious Diseases. Her research focuses on medical complications of substance use including HIV and hepatitis C virus (HCV). She is also interested in models of care and improving the continuum of care for individuals with HIV/ HCV. She is currently the Principal Investigator on a NIH K23 Mentored Career Development Award to improve linkage to care after testing for HIV and HCV at a drug detoxification center.
Sabrina A. Assoumou, MD, MPH, is Assistant Professor of Medicine at Boston University School of Medicine and an attending physician in the section of Infectious Diseases at Boston Medical Center. She is a clinician-investigator who provides care to human immunodeficiency virus (HIV)-infected patients at the BUSM/BMC’s Centers for Infectious Diseases. Her research focuses on medical complications of substance use including HIV and hepatitis C virus (HCV). She is also interested in models of care and improving the continuum of care for individuals with HIV/ HCV. She is currently the Principal Investigator on a NIH K23 Mentored Career Development Award to improve linkage to care after testing for HIV and HCV at a drug detoxification center.
Dr. Assoumou graduated magna cum laude from Williams College and obtained her medical degree from the University of Rochester School of Medicine and Dentistry. She then completed a combined Internal Medicine-Pediatrics residency at Brown University and an Infectious Diseases Fellowship at Harvard University’s Beth Israel Deaconess Medical Center where she was awarded the Finland Award for Research Excellence. She also earned a Master of Public Health at the Harvard T.H. Chan School of Public Health. Dr. Assoumou received an Excellence in Teaching Hospital-Based Faculty Award at BMC in 2017. She was also recognized as the Distinguished Faculty of the Month in April 2020 for her service to the BUSM community in teaching, activities on committees, and the mentoring of students, trainees, and junior faculty.
Data-Driven Youth-Led Health Promotion Strategy for a Safety Net Accountable Care Organization
The “Data-Driven Youth-Led Health Promotion Strategy for a Safety Net Accountable Care Organization” is a partnership between researchers at the Boston University School of Social Work and Boston Medical Center’s Department of Family Medicine. The overall goal of the project is to develop and vet a youth-driven health promotion campaign informed by a youth-led health assessment. The research will be guided by a youth advisory board consisting of six to eight youth of color who will be trained in the principles of youth participatory action research (YPAR), health equity, the social determinants of health, health promotion practice, and data-driven planning.

Astraea Augsberger is an Assistant Professor at Boston University School of Social Work. She earned her MSW and Ph.D. from Columbia University. She has over a decade of clinical practice experience with children, youth, and families in the child welfare, juvenile justice and mental health systems. Her research interests include youth civic engagement, youth development, child welfare policy and programs, and health equity. She employs community-engaged research, youth participatory action research, and in-depth qualitative research methods to elevate the voices of youth and communities in identifying research priorities and relevant solutions to community concerns. She is an affiliated faculty member of the Boston University Center for Innovation in Social Work & Health (CISWH) and the Boston University Initiative on Cities (IOC).
Dr. Katherine Gergen Barnett is the Vice-Chair of Primary Care Innovation & Transformation and the Program Director in the Department of Family Medicine at Boston Medical Center (BMC). Katherine joined BMC in 2009 after completing her residency and chief residency there. Prior to BMC, Dr. Gergen Barnett attended Yale University School of Medicine, worked at NIH, and completed a fellowship studying a model of group prenatal care for underserved women.
Dr. Gergen Barnett’s primary interests are behavioral health integration, preventive medicine, nutrition, mindfulness-based stress reduction, women’s health, and group care. Dr. Gergen Barnett’s research career has been primarily focused on innovative models of care to address chronic medical conditions, physician burnout, and engaging community partners in creating feasible solutions to increase health and wellness in urban communities.
“Immune Response and mEdical Complications of coVid-19 suRvivors (I-RECOVR) study”
Th
 Dr. Nahid Bhadelia is an infectious diseases physician and the Medical Director of Special Pathogens Unit at Boston University School of Medicine, a medical unit designed to care for patients with highly communicable diseases. She is an Associate Professor in the Section of Infectious Diseases. She oversees the medical response program for Boston University’s maximum containment Biosafety Level 4 program at National Emerging Infectious Diseases Laboratories.
Dr. Nahid Bhadelia is an infectious diseases physician and the Medical Director of Special Pathogens Unit at Boston University School of Medicine, a medical unit designed to care for patients with highly communicable diseases. She is an Associate Professor in the Section of Infectious Diseases. She oversees the medical response program for Boston University’s maximum containment Biosafety Level 4 program at National Emerging Infectious Diseases Laboratories.
During the West African Ebola epidemic, she served as a clinician in several Ebola treatment units, working with World Health Organization and Partners in Health. She currently serves as the clinical lead for the Joint Mobile Emerging Disease Intervention Clinical Capability (JMEDICC) program which is a joint US-Ugandan effort to create clinical research capacity to combat viral hemorrhagic fevers in Uganda at the border of Democratic Republic of Congo. She serves on national and interagency groups focused on medical countermeasures, the intersection between public health preparedness, research, and clinical care for emerging pathogens. Her research focuses on identification of safe and effective clinical interventions and infection control measures related to viral hemorrhagic fevers.
She has served as a subject matter expert to US Centers for Disease Control and Prevention, Department of Defense, Global Fund to Fight AIDS, Tuberculosis and Malaria, and World Bank.
Dr. Bhadelia is also an Assistant Professor at the Institute of Human Security at the Tufts Fletcher School of Law and Diplomacy, where she teaches a course on human security and emerging infectious diseases. She received her Doctorate of Medicine from Tufts University and completed her internal medicine residency and chief residency at Mount Sinai Hospital in New York. Her Infectious Diseases Fellowship was completed at Columbia Presbyterian Hospital.
PTH/PTHrP Receptor Signaling in Osteocytes during Aging
Dr. Divieti Pajevic’s research is focused on studying the effects of hormones, such as parathyroid hormone, and mechanical forces on bone and teeth. Recently, her group has been investigating the cross-talk between bone and muscle and the effects of aging. In her current CTSI proposal titled “PTH/PTHrP Receptor Signaling in Osteocytes During Aging,” Dr. Divieti Pajevic will use a combination of genetically modified mice and cell lines to investigate if PTH signaling in osteocytes alters skeletal progenitors and protects these cells from senescence.
Paola Divieti Pajevic, MD, PhD, is an Associate Professor of Translational Dental Medicine at the Goldman School of Dental Medicine at Boston University and the Director of the Bone Cells Core which is part of the MGH-Center for Skeletal Research.
Lionoleic Acid Promotes Pathogenic T Cells in Type 1 Diabetes
泰
Dr. Hans Dooms is an immunologist who currently holds a position as an Assistant Professor in Medicine and Microbiology at the Arthritis and Autoimmune Diseases Research Center of Boston University School of Medicine. He received his Ph.D. from Ghent University in Belgium and continued his training in immunology at the University of California San Francisco in the laboratory of Dr. Abul Abbas. His research interests are focused on the biology of T cells and their role in the autoimmune diseases Type 1 Diabetes and Systemic Sclerosis. His laboratory is currently studying how autoreactive T cells acquire and maintain their disease-causing properties and how these cells escape immune regulatory mechanisms aimed at preventing autoimmunity. Dr. Dooms has received funding for his research from NIH, JDRF, and the American Diabetes Association. He has authored 29 publications, many included in some of the most prestigious journals in the field of immunology.
Defining the Role of Hippo Pathway Inactivation in Melanomagenesis
Dr. Ganem’s CTSI award is focused on testing the hypothesis that inactivation of the Hippo tumor suppressor pathway plays an important role in melanoma development.
Neil J. Ganem is an Associate Professor in the Department of Pharmacology & Experimental Therapeutics, and Department of Medicine, Division of Hematology and Oncology. He directs the Laboratory of Cancer Cell Biology, where his team used a combination of high-resolution microscopy, genome-wide screening, bioinformatics, and animal model systems to understand the causes and consequences of genome instability in human cancer.
Primary Care Connection in Maine
心肌梗死
The research team for this project includes Anna Goldman, MD, MPA, MPH, of BUSM/BMC; Sarah Gordon, PhD, MPH, of BUSPH; and Benjamin Sommers, MD, PhD, of Harvard T.H. Chan School of Public Health.
 Anna L. Goldman MD, MPA, MPH, is a general internist practicing primary care, and a health services researcher. Her research centers on the effects of insurance and payment policies on care access for the poor and undeserved. She focuses on the health insurance programs established by the Affordable Care Act, the Medicaid expansion and Marketplace insurance. She also investigates the effects of accountable care organizations (ACOs) in the Medicaid program on health care quality and access. She has a medical degree from Mount Sinai School of Medicine, a Master’s Degree in Public Health from the Harvard T.H. Chan School of Public Health,and a Master’s Degree in Public Affairs from Brown University. She completed an internal medicine residency at Cambridge Health Alliance.
Anna L. Goldman MD, MPA, MPH, is a general internist practicing primary care, and a health services researcher. Her research centers on the effects of insurance and payment policies on care access for the poor and undeserved. She focuses on the health insurance programs established by the Affordable Care Act, the Medicaid expansion and Marketplace insurance. She also investigates the effects of accountable care organizations (ACOs) in the Medicaid program on health care quality and access. She has a medical degree from Mount Sinai School of Medicine, a Master’s Degree in Public Health from the Harvard T.H. Chan School of Public Health,and a Master’s Degree in Public Affairs from Brown University. She completed an internal medicine residency at Cambridge Health Alliance.

Sarah Gordon is an Assistant Professor in the Department of Health Law, Policy, and Management at the Boston University School of Public Health. Her research is dedicated to studying coverage and access to care among low-income populations, with a particular emphasis on Medicaid policy. Her work seeks to understand how the fragmentation of the U.S. health insurance system impacts utilization, quality, and continuity of care. Dr. Gordon recent projects leverage state-level datasets, such as all payer claims databases, and quasi-experimental study designs to evaluate the effects of state-level policies. She received her doctorate in Health Services Research from the Brown University School of Public Health and a M.S. in Social and Behavioral Sciences from the Harvard T. H. Chan School of Public Health
A Tool to Identify PrEP Eligible Youth in Primary Care
Pediatricians are the most common provider of primary care for youth, and frequently provide care to individuals through their early-to-mid 20s. Thus, pediatric primary care offers an opportune setting for identifying youth who are eligible for pre-exposure prophylaxis (PrEP). To successfully identify PrEP-eligible youth, it is essential that pediatricians ask valid, developmentally appropriate screening questions that resonate with youth and elicit accurate information. The objective of this proposal is to conduct mixed methods, translational research to develop (Aim 1), and conduct a pilot study (Aim 2) of a HIV risk assessment tool that pediatricians can integrate into their current primary care practice to identify PrEP-eligible youth. Data from the pilot study will directly inform a subsequent clinical trial of a comprehensive PrEP service delivery model for pediatric primary care.
Scott Hadland is a pediatrician and addiction specialist at Boston Medical Center and Boston University School of Medicine. He holds triple board certification in General Pediatrics, Adolescent Medicine, and Addiction Medicine. Dr. Hadland’s clinical and research interests focus on adolescent and young adult substance use disorder prevention and treatment. In the proposed work, Dr. Hadland will extend his work into human immunodeficiency virus (HIV) prevention among youth, with a goal of enhancing pre-exposure prophylaxis (PrEP) delivery in pediatric primary care.
Influenza Induced Lymphangiogenesis
The overall objective of this proposal is to identify lymphatic-centered strategies to contain viral lung infections. To achieve this goal, the PIs use a mouse influenza model that allows for deciphering the functional therapeutic properties and origins of the markedly expanded pulmonary lymphatic system that the PIs found accompanies influenza. Based on preliminary data, the central hypothesis is that new lymphatic growth facilitates immunomodulatory events and alterations in lymphatic cell function that are central to virus containment. The PIs propose to use the CTSI pilot grant to perform a detailed transcriptional analysis of lymphatic cells as the lung responds to influenza.
The PIs driving this project are Dr. Matthew Jones and Dr. Alan Fine. Together, they have a successful history of collaboration on various projects, grants, and papers.
Dr. Matthew Jones is a lung molecular biologist with an extensive history of studying the lung’s response to infection. Dr. Jones also brings a deep knowledge of gene expression analytical methodology to this project.
Dr. Alan Fine is a practicing pulmonologist with broad experience in the treatment of lung disease. He has a long history of external funding that supports work aimed at understanding the fundamental biology of lung cells, lung development, and disease mechanisms.
Clinical Pipeline for Single Cell Profiling of Triple Negative Breast Cancer
The research for this CTSI pilot award will recruit, consent, and invite BMC patients with breast cancer to participate in translational research, by providing biopsy specimens for single-cell RNA sequencing to answer cutting-edge research questions on the behavior of tumor cells and the immune environment.
 Dr. Naomi Ko is an Assistant Professor of Medicine at Boston University School of Medicine (BUSM) and a medical oncologist at Boston Medical Center (BMC). Her research is directed to understanding the root causes of cancer disparities, the disconnect between scientific discoveries in cancer treatment, and delivery of evidence-based treatment to vulnerable, racial/ethnic minority women with breast cancer. She is actively investigating how tumor biology, social, and treatment factors influence breast cancer outcomes in undeserved, diverse breast cancer populations.
Dr. Naomi Ko is an Assistant Professor of Medicine at Boston University School of Medicine (BUSM) and a medical oncologist at Boston Medical Center (BMC). Her research is directed to understanding the root causes of cancer disparities, the disconnect between scientific discoveries in cancer treatment, and delivery of evidence-based treatment to vulnerable, racial/ethnic minority women with breast cancer. She is actively investigating how tumor biology, social, and treatment factors influence breast cancer outcomes in undeserved, diverse breast cancer populations.
Single-Cell Analysis of AL Amyloidosis Plasma Cells
如果
遗传算法
Machine Learning Risk Stratification of Stroke in Patients with AF
We aim to develop a clinically-oriented risk prediction model of stroke in patients with non-valvular atrial fibrillation using novel, clinically-oriented machine learning methods and data from Boston Medical Center and the UK Biobank
否
Ch
Soft Robotic Platform for Restoring Haptic Feedback in Robotic Surgery
Robotic surgery has improved minimally invasive surgical procedures and shortened learning curves for surgeons. However, during robot-assisted procedures, haptic feedback is not available to the surgeon, resulting in uncontrolled force application which can lead to intraoperative complications. Absent haptic feedback is reputed to be among the reasons that impede further spread of surgical robots.
The capability to sense and touch the anatomy realistically has the potential to increase safety and precision during robotic surgery, with better patient outcomes. In this proposal, we will focus on restoring haptic feedback in minimally invasive robotic surgery by developing a soft robotic platform consisting of a soft sensing unit, to monitor forces exerted during surgical tasks and transduce this information to a soft actuation unit, that will map tactile information back to the surgeon.
Sheila Russo is an Assistant Professor in the Department of Mechanical Engineering and the Division of Materials Science and Engineering at Boston University. Prof. Russo completed her Ph.D. degree at the BioRobotics Institute, Sant’Anna School of Advanced Studies, Italy. She completed her postdoctoral training at the Harvard John A. Paulson School of Engineering and Applied Sciences and the Wyss Institute for Biologically Inspired Engineering. She is the founder and director of the Material Robotics Laboratory at BU that aims at bridging the gap between material science and robotics, and focuses on design, mechanics, and manufacturing of novel multi-scale and multi-material biomedical robotic systems. Her research interests include medical and surgical robotics, soft robotics, sensing and actuation, meso- and micro-scale manufacturing techniques, and advanced materials.
Pre-Clinical Validation of Novel Gene Editing Approaches for Sickle Cell Disease
With the support of the CTSI pilot grant, Dr. Vanuytsel will further develop the sickle cell disease (SCD)-specific iPSC platform as a pre-clinical tool to validate the efficacy of novel therapeutic gene editing strategies across a diverse SCD patient population.
Although SCD patients all share the same point mutation in the beta globin gene, the surrounding genetic background determines the severity of their disease course and response to therapy. Currently, several gene editing strategies are being explored as potentially curative approaches in clinical and pre-clinical trials. While these developments are truly exciting, their safety and efficacy will require thorough validation across a variety of SCD backgrounds to make sure that an effective treatment can be assured for every patient. iPSCs present an unlimited source of material that can be differentiated into erythroblasts that capture the exact genetic background of a patient, and thus make an excellent screening tool that could help predict the safety and efficacy of a particular therapeutic approach in a given genetic background prior to engaging in costly and invasive treatments.
Kim Vanuytsel is a Research Assistant Professor in the Division of Hematology and Medical Oncology at Boston University School of Medicine and the Center for Regenerative Medicine (CReM). She obtained a PhD in Stem Cell and Molecular Medicine from the Katholieke Universiteit Leuven (KULeuven) in Belgium. As a postdoc in Dr. George J Murphy’s lab, she developed novel resources and tools to better understand and treat blood disorders. Her current research focuses on finding better solutions for sickle cell disease (SCD) patients by using patient-specific induced pluripotent stem cells (iPSCs) as a versatile platform to study the disease, screen for compounds that can ameliorate the condition, and explore potential curative gene editing approaches.