Pathology Research
The pathological research department at the BU CTE Center, operates the UNITE Brain Bank and BU ADRC Brain Bank, and works closely with the Framingham Heart Study (FHS) Brain Bank and Department of Veterans Affairs Biorepository Brain Bank (VABBB) to study tissue and fluid samples from brain donors. Our neuropathologists examine frozen and fixed tissue for genetic, molecular, and other projects, and to provide UNITE and BU ADRC brain donor families with diagnoses. They work to publicize the findings and maintain the storage of the tissue and samples.
UNITE Brain Bank
The UNITE Brain Bank is the largest tissue repository in the world focused on traumatic brain injuries (TBI) and chronic traumatic encephalopathy (CTE). The brain bank collects central nervous system tissue samples (brain, spinal cord and eyes) from deceased athletes, military veterans, and others to better understand the long-term effects of repetitive head impacts, TBI and CTE. Ann McKee, MD and her team of neuropathologists, clinicians, and investigators have published more than 175 peer-reviewed papers and studies focused on CTE in highly regarded journals. They’ve also written more than 30 grants to support the daily operations of the brain bank.
Team
-

Ann McKee
William Fairfield Warren Distinguished Professor of Neurology and Pathology, Boston University Chobanian & Avedisian School of Medicine
Director, BU Alzheimer’s Disease Research Center & BU CTE Center
Chief of Neuropathology, VA Boston Healthcare System -

Jon Cherry
Director, Digital Core, BU ADRC & BU CTE Center
Research Neuropathologist, BU ADRC & BU CTE Center
Assistant Professor of Pathology and Laboratory Medicine, Boston University Avedisian & Chobanian School of Medicine
Research Health Scientist, VA Boston Healthcare System -

Thor Stein
Director, Molecular Research, BU CTE Center
Director, Neuropathology Core, BU ADRC
Associate Professor of Pathology and Laboratory Medicine, Boston University Avedisian & Chobanian School of Medicine
Neuropathologist, VA Boston Healthcare System -

Victor Alvarez
Director of Laboratory Operations, BU CTE Center
Assistant Professor of Neurology, Boston University Chobanian & Avedisian School of Medicine
Meet the UNITE Study Research Faculty
Post-Docs
Doctoral Students
Histologists
Brain Processing Staff
Research Assistants & Administrative Staff
Pathology Images
Source: Structural MRI profiles and tau correlates of atrophy in autopsy-confirmed CTE
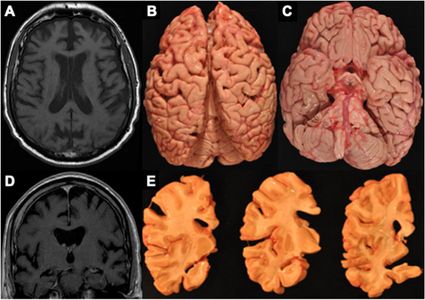
Figure 4. MRI and autopsy patterns of atrophy and P-tau deposition in a brain donor with CTE
The figure shows an antemortem axial (A) and coronal (D) T1 MRI sequence and corresponding gross atrophy at autopsy (B, C, E) of a male former professional American football player with CTE stage IV. The antemortem MRI scan was done when he was in his early 60’s and he died in his mid-70s and donated his brain. The antemortem MRI and neuropathological examination both showed frontal and temporal cortical atrophy (A–E) along with atrophy of medial temporal lobe structures (C, E) including the hippocampus and amygdala.
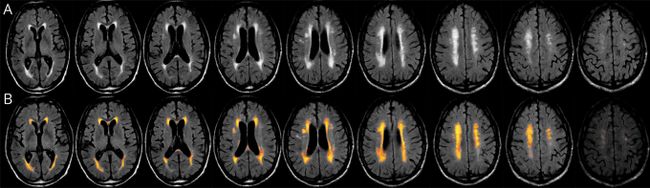
Figure 1. Example of FLAIR and Lesion Segmentation From the Lesion Prediction Algorithm of the Lesion Segmentation Toolbox
Fluid-attenuated inversion recovery (FLAIR) scan of a mid-70-year-old former professional American football player with autopsy-confirmed stage III/IV chronic traumatic encephalopathy. White matter hyperintensities were quantified using log-transformed values for the total lesion volume, calculated using the lesion prediction algorithm (LPA) from the Lesion Segmentation Toolbox. The LPA segments lesions in new images by providing an estimate for the lesion probability for each voxel. Total lesion volume in milliliters is extracted by thresholding derived lesion probability maps at a threshold kappa of 0.5 (default LPA recommendation) and only lesions with volume >0.015 mL are counted. The top row (A) contains the native FLAIR sequence and the bottom row (B) contains the corresponding lesion belief maps overlaid on the FLAIR with lesion threshold at 0.5 using Slicer.
Source: TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy
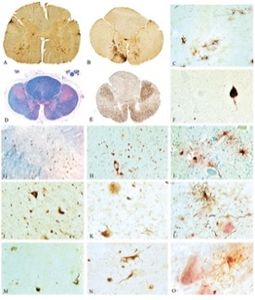
FIGURE 2
Sp
To request access to biological samples from the UNITE Brain Bank, please review our data request process.




























